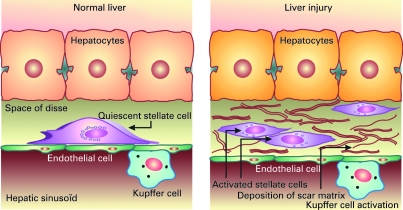
Figure 3.
The stellate cell: a key cell implicated in fibrogenesis. Hepatic stellate cells (HSCs) exist in the space between parenchymal cells and sinusoidal endothelial cells of the hepatic lobule, and store vitamin A as retinyl palmitate in lipid droplets in the cytoplasm. In physiological conditions, these cells play pivotal roles in the regulation of vitamin A homeostasis; they express specific receptors for retinol-binding protein (RBP), a binding protein specific for retinol, on their cell surface, and take up the complex of retinol and RBP by receptor-mediated endocytosis. In a normal state, HSCs appear as quiescent vitamin A-storing cells. When activated via several stimuli (infection, alcohol, cytokines, etc.) they acquire a proliferative myofibroblast phenotype. In pathological conditions such as chronic hepatitis C, HSCs lose vitamin A and synthesise a large amount of extracellular matrix components including collagen, proteoglycan and adhesive glycoproteins. Kupffer cells, the resident liver macrophages, remove material from the portal circulation. Kupffer cells may act both as effector cells in the destruction of hepatocytes by producing harmful soluble mediators and as antigen-presenting cells during viral infections of the liver. Moreover, they may represent a significant source of chemoattractant molecules for cytotoxic CD8 and regulatory T cells. Their role in fibrosis is well established as they are one of the main sources of transforming growth factor β1 production, which leads to the transformation of HSCs into myofibroblasts.