Abstract
The adductor canal is a conical or pyramid-shaped pathway that contains the femoral vessels, saphenous nerve and a varying amount of fibrous tissue. It is involved in adductor canal syndrome, a claudication syndrome involving young individuals. Our objective was to study modifications induced by aging on the connective tissue and to correlate them to the proposed pathophysiological mechanism. The bilateral adductor canals and femoral vessels of four adult and five fetal specimens were removed en bloc and analyzed. Sections 12 µm thick were obtained and the connective tissue studied with Sirius Red, Verhoeff, Weigert and Azo stains. Scanning electron microscopy (SEM) photomicrographs of the surfaces of each adductor canal were also analyzed. Findings were homogeneous inside each group. The connective tissue of the canal was continuous with the outer layer of the vessels in both groups. The pattern of concentric, thick collagen type I bundles in fetal specimens was replaced by a diffuse network of compact collagen bundles with several transversal fibers and an impressive content of collagen III fibers. Elastic fibers in adults were not concentrated in the thick bundles but dispersed in line with the transversal fiber system. A dynamic compression mechanism with or without an evident constricting fibrous band has been proposed previously for adductor canal syndrome, possibly involving the connective tissue inside the canal. The vessels may not slide freely during movement. These age-related modifications in normal individuals may represent necessary conditions for this syndrome to develop.
Keywords: anatomy, electron, extremity, intermittent claudication, microscopy, polarization, regional, scanning
Introduction
Neurovascular bundles are often located inside conical or tubular pathways in the human body, which are muscular, osseous, fibrous or mixed in nature. Examples include the carpal and tarsal tunnels and the cranial foramina. These bundles and their corresponding adjacent pathways are well-known by physicians and even medical and anatomy students due to significant clinical implications. This trend has become more evident in recent years since the propagation of ‘problem-based learning’ which correlates clinical situations at an early stage during anatomy teaching, frequently at the pre-medical level. Several of these clinical syndromes involving entrapment of neurovascular bundles have long been recognized, and are responsible for the coining of some of the best-known historical and clinical vignettes of medicine, used to renew interest and facilitate anatomy teaching. The jugular foramen, superior orbital fissure, ulnar, carpal tunnel and thoracic outlet syndromes are just a few examples of these conditions. In fact, frequently the clinical importance of these conditions has spurred the study of local anatomy.
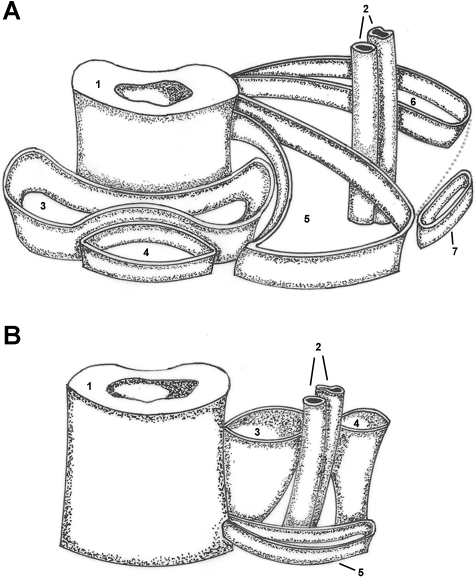
For the above-mentioned reasons, the adductor, or Hunter's, canal is relatively well-known as an anatomical entity, although few physicians are sufficiently aware of the clinical syndromes that may involve it, let alone have a thorough understanding of its anatomy. The adductor canal was first described by John Hunter in 1786 and its gross anatomical features were delineated in the following years. Mid-20th century anatomists such as Novotny (1950), Annersten (1951) and Palma (1952) focused on the physiology of the contents of the adductor canal during motion and exercise. The adductor canal has been classically described as being conical or pyramid-shaped and contains in its interior the femoral vessels and saphenous nerve along with a varying amount of fibrous tissue. Further research has suggested a proximal pyramid-like base with a tubular apex distally at the adductor hiatus, its narrowest point (De Souza et al. 1978), being limited on three sides by the vastoadductor membrane and sartorius muscle (antero-medial), vastus medialis (antero-lateral) and adductor magnus and longus (posterior) muscles as its most accepted limits (Fig. 1) (Testut & Jacob, 1956; De Souza et al. 1978, 1984; Tubbs et al. 2007).
Fig. 1.
Schematic diagram depicting the topography of the adductor canal (A) and its hiatus (B). (A)1 – femur; 2 – femoral vessels; 3 – vastus intermedius muscle; 4 – rectus femuris muscle; 5 – vastus medialis muscle; 6 – adductor magnus muscle; 7 – sartorius muscle; dotted line – vastoadductor membrane; (b)1 – femur; 2 – femoral vessels; 3 – adductor magnus muscle, oblique fibers; 4 – adductor magnus muscle, longitudinal fibers; 5 – vastus medialis muscle.
Two clinical syndromes are related to compression of the neurovascular bundle inside the adductor canal. The compression of the saphenous nerve at the adductor hiatus is believed to cause pain on the medial aspect of the knee. Some orthopedic surgeons note that saphenous nerve neuralgia should be remembered as a differential diagnosis of pain referred to the knee region but it is so infrequent that diagnosis is rarely made (Morganti et al. 2002). In specific cases, saphenous nerve block alone or combined with surgical exploration and decompression satisfactorily relieves symptoms, especially in cases with an evident compression at the hiatus (Luerssen et al. 1983). However, the vascular syndrome related to arterial compression is much more frequent than saphenous nerve neuralgia and is remembered as the ‘classical’ feature associated with compression at the adductor canal, although in rare situations both can be present simultaneously. It presents itself as a claudication syndrome during vigorous exercise, typically in young males in their late 20s and 30s, which led some authors to include the adjacent hypertrophied muscles in the proposed pathophysiological mechanism (Pratt, 1971). Further investigation with lower limb arteriography sometimes reveals a short, probably extrinsic, compression of the femoral artery in the distal thigh with few or absolutely no other signs of atherosclerotic lesions at other sites. It is, however, not infrequent for the arteriography to be considered normal, which points towards a dynamic mechanism. Definite diagnosis is made during surgical exploration when a fibrous band is encountered in the interior of the adductor canal or, most frequently, the adductor hiatus encroaches on the femoral artery. Section of the fibrous band and/or adductor hiatus, coupled or not with an autologous or synthetic femoral artery graft should promptly resolve claudication symptoms. Other findings which may also be present reveal tunica intima proliferation and tears on the stenosed segment, which researchers also attribute to long-standing, dynamic extrinsic compression (Vitiello, 1975; Balaji & DeWeese, 1984; Verta et al. 1984; Scholten et al. 1993; Walsh et al. 1997).
Several hypotheses were raised from the 1950s onwards which have tried to explain this exquisite claudication syndrome. The common point of most of these theories is the connective tissue of the adductor canal. Some sort of constricting fibrous band and the interaction between adjacent structures and the vessels have been implicated in almost all of these theories. On the other hand, morphological studies about the connective tissue of the canal are scarce. Particularly, none of these studies addresses the question of why individuals do not develop early symptoms (e.g. during childhood) if connective tissue abnormalities and/or structural alterations are congenital or developmental, as suggested in most theories. A possible answer is that the normal aging process might involve connective tissue modifications that affect the normal vessel–canal wall interaction, thus leading to symptomatology in susceptible individuals. However, the normal aging process of the connective tissue of the canal has never been described from a structural and ultrastructural standpoint (De Souza et al. 1984). Therefore the objective of this study was to perform a detailed qualitative morphological analysis of the perivascular fibrous tissue of the femoral vessels inside the adductor canal. To test the hypothesis that this fibrous tissue undergoes modifications in early adulthood as part of a physiological aging mechanism both fetuses and adult cadavers were studied with optical and scanning electron microscopy methods.
Materials and methods
Both inferior limbs of five human fetuses (4–6 months intrauterine age, two male and three female specimens) and four adult cadavers (three male, one female – aged 19, 26, 41, 47 years) were utilized in this study. None of the subjects had signs of previous trauma or surgery involving the inferior limbs. These specimens were part of the collection of the Department of Anatomy, Instituto de Ciencias Biomedicas da Universidade de Sao Paulo and had been subjected to fixation in 4% formaldehyde for at least 6 months post mortem.
After the removal of the skin flap, dissection initially revealed both the popliteal artery and vein in their fossa. These vessels were followed proximally up to the beginning of the adductor canal. In adult specimens, superficial muscles of the thigh were removed, exposing the contents of the adductor canal. The femoral artery and vein were removed en bloc together with the perivascular fibrous tissue and a layer of the surrounding muscles, thus preserving the relationship with its neighboring structures. These tissue blocks were embedded in Paraplast® (Paraplast, McCormick Scientific, St. Louis, MO) and 12-µm-thick sections were obtained in the microtome. Sections were then stained for a complete histological analysis of the collagen fibers with Sirius red and Azo dyes (Junqueira et al. 1979). Elastic fibers were examined with the Verhoeff (Verhoeff, 1908) and Weigert (Weigert, 1898) methods; Weigert-stained sections were also counter-stained with Giemsa. Photomicrographs were made under normal and polarized (Sirius red) light at different magnifications (Axioshop40, Zeiss).
Sections 40 µm thick from two specimens in each group were also obtained and post-fixed with 1% osmium tetroxide, following by dehydration in growing series of alcohols. These specimens were critical point-dried (Bal-tec CPD-030, Liechtenstein), mounted on appropriate stubs, gold-coated in a Bal-tec SCD-040 ion sputterer and examined in a Jeol JSM-6100 (Tokyo, Japan) scanning electron microscope.
Results
Morphology findings were fairly homogeneous among all specimens in each group.
Fetuses – light microscopy
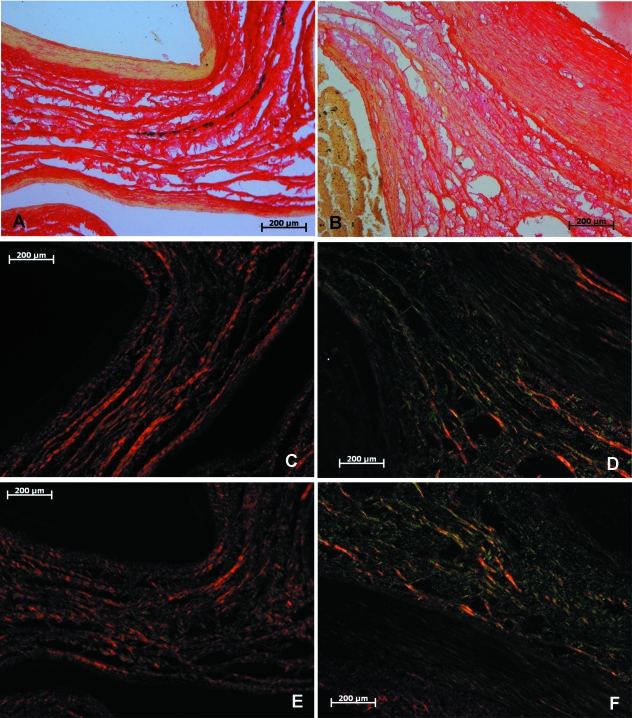
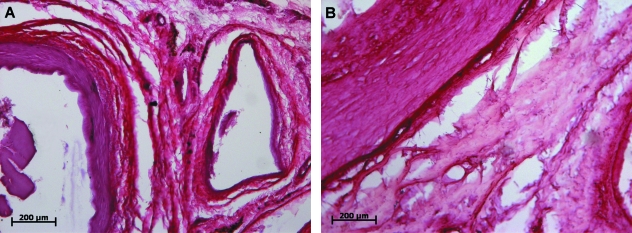
Sirius red-staining of the connective tissue inside the adductor canal of fetuses revealed an intricate network of collagen fibers. Collagen bundles between the femoral artery and vein were arranged in a predominantly concentric pattern with smaller bundles traversing the space between them. These bundles were not particularly compact but were arranged in a loose manner (Fig. 2A,C,E). Examining these slides under polarized light revealed a predominance of type I collagen bundles (Fig. 2C,E) with a small amount of type III collagen in the fine transversal fibers. Collagen fibers were further evidenced using Azo staining (Fig. 3A), which confirms the concentric arrangement around the femoral vessels. Some adipose cell clusters were also evident and characteristic of the younger specimens (Fig. 3A).
Fig. 2.
Sirius red-stained sections. Fetal (A,C,E) and adult (B,D,F) specimens, under normal (A,B) and polarized (C,F) light. The described pattern of connective tissue organized in concentric bundles is evident in the fetal sections; a denser, disorganized pattern is observed in adult specimens, as well as an increased content of type III collagen fibers.
Fig. 3.
Azo-stained sections, fetal (A) and adult (B) specimens. Confirmation of Sirius red findings. Adipose cells present in the center of the section. Evident concentric pattern in fetal specimen compared to dense pattern in adult canal.
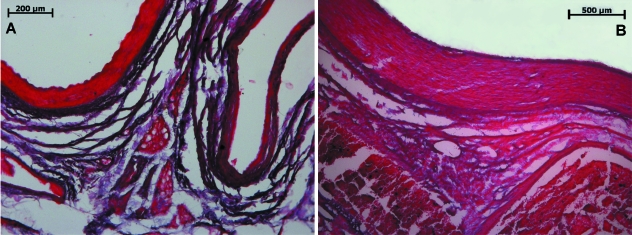
Elastic fibers were analyzed with the Verhoeff and Weigert techniques. Apart from the expected elastic fibers concentrated on the vessel walls, especially the femoral artery, these fibers were evident inside the concentric connective tissue bundles (Figs 4A and 5A). Only a small number of elastic fibers in the transversal intertwining fibers were observed.
Fig. 4.
Verhoeff-stained sections, fetal (A) and adult (B) specimens. Elastic fiber content greatly diminished in adult specimens; note concentric arrangement in fetal specimen.
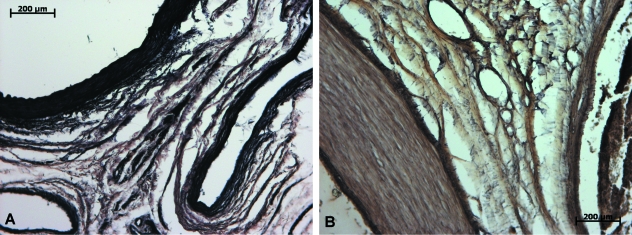
Fig. 5.
Weigert-stained sections, fetal (A) and adult (B) specimens. Concentric arrangement of collagen fibers in fetuses and the presence of transversally oriented elastic fibers in the adult specimen.
Adults – light microscopy
There were interesting differences in the results of light microscopy analysis in adult subjects. Sirius red-staining also evidenced some collagen bundles between the artery, vein and adjacent muscular walls but not nearly as grouped as in fetuses. In fact, connective tissue was much more compact and intertwining fibers abundant. This was the predominant feature, to the point that in some sectors the connective tissue was a dense mass and bundles are absent (Fig. 2B,D,F),
The Azo stain depicted significant amounts of connective tissue between the vessels and muscular walls (Fig. 3B), although the organization into larger bundles was lost altogether. Further investigation employing polarized light and Sirius red-stained sections delineated a massive increase in type III collagen in the transversal fibers between the few remaining type I bundles (Fig. 2D,F). It is also noticeable that this process involved not only a simple substitution of type I for type III collagen fibers; thick concentric bundles were no longer the preponderant feature but rather a pattern of more delicate type III collagen fibers organized in several directions in the midst of the denser connective tissue. Elastic fiber analysis through Verhoeff and Weigert stains also showed distinct characteristics – elastic fibers were much scarcer in adult specimens, lightly dispersed among the connective tissue situated between femoral vessels and muscular walls. Only a small proportion of elastic fibers in both Weigert- and Verhoeff-stained sections were located in concentric bundles (Figs 4B and 5B).
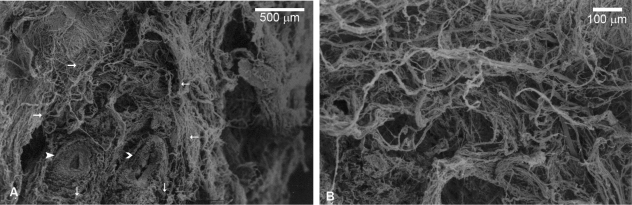
Fetuses – scanning electron microscopy
Low-power SEM clearly demonstrated the triangular section of the adductor canal with the femoral artery and vein in its interior (Fig. 6A). It also confirmed the loose arrangement of the connective tissue surrounding these vessels; significant space was evident even in low-power micrographs. The apparently concentric or circular arrangement delineated in light microscopy analysis was evident near the vessels, although this arrangement was lost near the muscular walls. High-power magnification further delineated the space between connective tissue. This can be estimated to be of at least the same relative diameter of the bundles (Fig. 6B), which lies in the 10-µm range.
Fig. 6.
Scanning electron microscopy (SEM) photomicrographs, fetal specimen. Low-power (A) photomicrograph shows evidence of the triangular nature of the adductor canal (small arrows) with the femoral artery (closed arrowhead) and vein (open arrowhead). High-power photomicrograph (B) demonstrates considerable space between collagen bundles of at least the same relative diameter of the bundles.
Adults – scanning electron microscopy
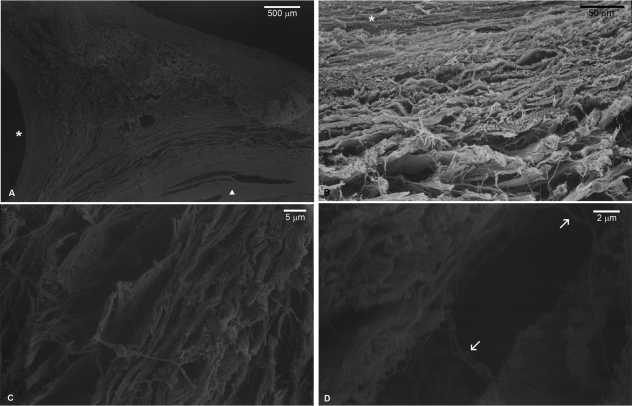
Results from the light microscopy analysis suggested that the circular arrangement of the connective tissues is lost altogether in adult specimens. However, SEM analysis showed that it is at least partially preserved in the region immediately adjacent to the vessels (Fig. 7A). This demonstrates that the connective tissue of the adductor canal is in fact attached to the outer layers of the vessels and a transition zone exists (Fig. 7B). There was not, however, a general arrangement pattern such as the concentric features of fetal specimens. Higher-power SEM evidenced the connective tissue bundles in a much ‘tighter’ arrangement than the fetal specimens (Fig. 7C). Further increase in magnification revealed that not only the arrangement was much more compact but there were also fine intertwining fibers spanning the space between the bundles (Fig. 7D).
Fig. 7.
SEM photomicrographs, adult specimens. A low-power photomicrograph (A) demonstrates a general disposition considerably denser than fetal specimens arranged around the femoral artery wall (triangle) and vein (asterisk). In (B) the transition zone from the artery wall (asterisk) to the canal connective tissue is evident; there is no clear distinction between the artery wall and the adjacent connective tissue but a transition zone. (C) High-power photomicrograph demonstrating a denser pattern; compare with Fig. 6B considering the relative size of the adductor canal in each group. (D) Intertwining fibers traversing the space between small collagen bundles (arrows).
Discussion
Most authors from the mid-20th century describe or comment on a ‘scissors-like’ compression mechanism during exercise over the femoral artery at the adductor hiatus, while also describing some sort of fibrous band or structure compressing the contents of the canal (Novotny, 1950; Annersten, 1951; Palma, 1952; Mayone & Palma, 1964). In both proposed pathophysiological mechanisms the importance of the perivascular fibrous tissue is evident – it could either be part of the compressing structure or, under certain circumstances after repeated local trauma, undergo hypertrophy and adhere to adjacent structures so that normal vessel movement during flexion and extension of the leg is impossible (Balaji & DeWeese, 1984). Even though case reports and reviews on adductor canal syndrome are abundant, no ultrastructural studies on the normal aspect and relations of the perivascular fibrous tissue of the adductor canal could be found (Tubbs et al. 2007). It would not be surprising if perivascular tissue in asymptomatic adult individuals exhibited some sort of adherence to the vessels and adjacent muscles or the adductor hiatus rather than functioning solely as a ‘buffer’ or ‘cushion’ during motion.
Morphological modifications were obvious when comparing the adductor canal in fetal and adult specimens. The loss of the concentric collagen bundle pattern and the increase in transversal fiber density and type III collagen proportion could be satisfactorily demonstrated with the staining techniques employed in this study. There is considerable debate about the ‘ideal’ morphological method to study and demonstrate collagen fibers. Optical microscopy coupled with such staining techniques as Sirius red, Azo and Masson trichrome (among many others), transmission electron microscopy (TEM) and immunohistochemistry have all been used to delineate collagen fibers successfully. The decision to employ Sirius red staining was made based on the comparatively large size of the adductor canal – relatively low-power magnification could be used, whereas immunohistochemistry and TEM would be feasible and ideal only in smaller specimens under high-power magnification. Furthermore, the gross morphology of the connective tissue inside the adductor canal as well as the orientation of collagen bundles were also to be analyzed, and Sirius red is a classical and time-honored method of evaluating these characteristics.
The ultimate clinical significance of these modifications cannot be addressed here; however, a direct correlation to the loss of the physiologic scissors-like sliding mechanism proposed by some earlier anatomists may be imagined. The abundance of transversal fibers as exhibited in the adult specimens may confer a stronger attachment between the vessels and adjoining muscular walls of the adductor canal. The concomitant massive loss of the elastic fiber content would further add to this mechanism. The paucity of adipose tissue inside the canal is also a hallmark of the adult specimens but is already expected from gross morphological examination of specimens. Certainly the vast majority of adults undergo these changes without ever developing any symptoms; perhaps a study of the connective tissue of the adductor canal in affected individuals could reveal the far spectrum of these fiber changes which cause arterial compression and symptomatology.
SEM further delineated these alterations. While femoral vessels are loosely attached to the perivascular tissue in fetal specimens, SEM analysis demonstrated that perivascular collagen and elastic fibers possess a closer relation to the tunica externa of the vessels, especially the femoral artery. The strength of these attachments and how much they interfere with the proposed physiological ‘scissors-like’ mechanism is open to speculation but they are difficult to simply ignore. A possible explanation is that movement restriction at the vessel–perivascular tissue interface is greater than originally thought; in adult and older individuals its function as a movement buffer may be diminished in comparison with children. SEM analysis was also instrumental in demonstrating the relationship between thicker collagen bundles and transversal fibers as depicted in Fig. 7C and D.
Morphological alterations evidenced in presumably ‘normal’ or asymptomatic individuals should be viewed with caution. They should be considered part of the normal aging process and may be no more than a predisposing factor for the development of claudication and/or neuralgia symptoms. On the other hand, it is tempting to imagine that these characteristics may underlie the fact that infants and adolescents are not affected; perhaps only subtle additional alterations, such as a fibrous band, minor trauma or discrete alteration of the structure of the adductor hiatus, may be sufficient for the development of the full spectrum of the syndrome. Additional studies utilizing the same methodology on affected individuals undergoing surgery would certainly be welcomed.
References
- Annersten S. Effect of sympatectomy of the femoral artery in the adductor canal. Acta Chirur Scand. 1951;101:262–268. [PubMed] [Google Scholar]
- Balaji MR, DeWeese JA. Adductor canal outlet syndrome. JAMA. 1984;245:167–170. [PubMed] [Google Scholar]
- De Souza RR, Ferraz de Carvalho CA, König Júnior B. Anatomia Topográfica do canal adutor: forma, limites e constituição de suas paredes. Rev Paul Med. 1978;92:6–9. [PubMed] [Google Scholar]
- De Souza RR, Ferraz de Carvalho CA, Merluzzi Filho TJ, Vieira JAA. Functional anatomy of the perivascular tissue in the adductor canal. Gegenbaurs Morph Jahrb. 1984;130:733–738. [PubMed] [Google Scholar]
- Junqueira LCU, Bignolas G, Bretani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochemistry. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Luerssen TG, Campbell RL, Defalque RJ, Worth RM. Spontaneous saphenous neuralgia. Neurosugery. 1983;13:238–241. doi: 10.1227/00006123-198309000-00004. [DOI] [PubMed] [Google Scholar]
- Maymone S, Palma A. Ricerche anatomo-chirurgiche sulla strutura e sui reporti della guaina dei vasi femorali nel canale addutório. Ric Morf. 1964;30:129–145. [Google Scholar]
- Morganti CM, McFarland EG, Cosgarea AJ. Saphenous neuritis: a poorly understood cause of medial knee pain. J Am Acad Orthop Surg. 2002;10:130–137. doi: 10.5435/00124635-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Novotny H. Splitting of the adductor canal – a method of improving locally collateral circulation in thromboangeitis obliterans. Acta Chir Scand. 1950;99:332–340. [Google Scholar]
- Palma EC. Stenosed arteriopathy of Hunter canal and loop of adductor magnus. Am J Surg. 1952;83:723–733. doi: 10.1016/0002-9610(52)90174-8. [DOI] [PubMed] [Google Scholar]
- Pratt GH. Femoral artery compression (the adductor canal syndrome): surgical treatment. J Am Geriatr Soc. 1971;19:788–792. doi: 10.1111/j.1532-5415.1971.tb02429.x. [DOI] [PubMed] [Google Scholar]
- Scholten FG, Warnars GAO, Mali WPTM, Leeuwen MS. Femoropopliteal oclusions and the adductor canal hiatus, duplex study. Eur J Vasc Surg. 1993;7:680–683. doi: 10.1016/s0950-821x(05)80716-9. [DOI] [PubMed] [Google Scholar]
- Testut L, Jacob O. Miembro Inferior. In: Testut L, Jacob O, editors. Tratado de Anatomia Topográfica com aplicaciones medicoquirúrgicas. Barcelona: Salvat Editores; 1956. pp. 979–993. [Google Scholar]
- Tubbs RS, Loukas M, Shoja MM, Apaydin N, Oakes WJ, Salter EG. Anatomical and potential clinical significance of the vastoadductor membrane. Surg Radiol Anat. 2007;29:569–573. doi: 10.1007/s00276-007-0230-4. [DOI] [PubMed] [Google Scholar]
- Verhoeff FH. Some new staining methods of wide applicability including a rapid diferential stain for elastic tissue. J Am Med Assoc. 1908;50:876–877. [Google Scholar]
- Verta MJ, Vitello J, Fuller J. Adductor Canal Compression Syndrome. Arch Surg. 1984;119:345–346. doi: 10.1001/archsurg.1984.01390150075018. [DOI] [PubMed] [Google Scholar]
- Vitielo FS. Precisazioni anatomo-chirurgiche sulla morfologia nel canale di Hunter. Minerva Med. 1975;66:706–710. [PubMed] [Google Scholar]
- Walsh DB, Powell RJ, Strukel TA, Henderson EL, Cronenwett JL. Superficial femoral artery stenoses: characteristics of progressing lesions. J Vasc Surg. 1997;25:512–521. doi: 10.1016/s0741-5214(97)70262-3. [DOI] [PubMed] [Google Scholar]
- Weigert C. Ube reine Methode zur Forbung elastischer Fasern. Zentralbl Allg Pathol Pathol Anat. 1898;9:289–292. [Google Scholar]