Abstract
The L-type calcium channel plays a pivotal role in the regulation of a wide range of cellular processes, including membrane excitability, Ca2+ homeostasis, protein phosphorylation, and gene regulation. Alterations in the density or function of the L-type calcium channel have been implicated in a variety of cardiovascular diseases. Our previous study found that acute restraint stress could cause an enhancement of the L-type calcium current (ICa-L), which correlated with an up-regulation of activation characters of the calcium channel. In this study, we observed the change of ICa-L in rat ventricular myocytes under chronic restraint stress using the whole-cell patch-clamp technique and further explored its modulation mechanisms. The results showed that chronic restraint stress could also enhance ICa-L, but increased ICa-L was not accompanied by an alteration of the characteristics of activation and inactivation of the L-type calcium channel. Furthermore, results from reverse-transcription polymerase chain reaction and Northern blot showed that the abundance of α1c subunit messenger RNA of the L-type calcium channel in the ventricle was increased significantly after chronic stress, and Western blot analysis revealed the amount of α1c subunit protein also was elevated. These results suggest that the L-type calcium channel is involved in stress-induced cardiomyocyte injury, and the up-regulated expression of the L-type calcium channel α1c subunit might contribute to the ICa-L change under chronic stress, which is different from the regulation mechanism of acute restraint stress that mostly relates to an alteration in protein kinase A-dependent channel activation. Thus, it would provide a new insight into the mechanism of cardiomyocyte injury induced by stress.
Keywords: Calcium current, L-type calcium channel, Patch-clamp technique, Stress, Ventricular myocyte
Introduction
Stress is defined as an adaptive physiological response to the disruption of homeostasis. It has been proven that the effects of stress on organisms are paradoxical; that is, a moderate stress load can invoke protection, but a stress overload can cause injury or can contribute to diseases, including diabetes, gastric ulcer, obesity, cancer, and Parkinson’s disease. In particular, the relationship between stress and the risk of cardiovascular disease has been reported. Numerous studies have shown that the cardiovascular system is the major organ targeted by stress, and some scientists even consider that stress is the most important etiologic factor of cardiovascular diseases, such as hypertension, atherosclerosis, and even sudden cardiac failure (Das et al. 1999; Schwartz et al. 2003). Restraint is considered to be a nonspecific stressor, which can lead to a series of common biochemical and physiological changes. These changes are mediated by the neuroendocrine system including activation of hypothalamic–pituitary–adrenocortical axis and the sympathetic–adrenomedullary system and after over-secretion of corticosterone and catecholamine. Hence, the animal model of restraint stress is often used to study the influence of stress on physiological function and pathological processes. In our previous study (Zhao et al. 2007), it was demonstrated that restraint stress may lead to cardiac dysfunction and to structural injury of the heart. Moreover, severe cardiomyocyte apoptosis and necrosis were also found after restraint stress. Cardiomyocyte death is considered an important cellular basis for stress-induced cardiovascular injury and disease (Feuerstein and Young 2000). The underlying pathological mechanisms for cardiomyocyte damage caused by stress remain unclear, however.
It is well known that the L-type calcium channel plays a major role in determining the normal physiological function characteristics of cardiac myocytes. The depolarizing current through the L-type calcium channel contributes to the plateau phase of the cardiac action potential and to pacemaker activity in nodal cells. The influx of Ca2+ ions through the L-type calcium channel is crucial for excitation–contraction coupling in the heart (Bers 2002; Bodi et al. 2005). This influx of Ca2+ triggers the release of intracellular stores of Ca2+ from the sarcoplasmic reticulum, and the ensuing intracellular Ca2+ transient results in activation of the myofilaments. The L-type calcium channel can also impact other cellular processes modulated by intracellular Ca2+ such as gene expression and excitation–secretion coupling. Alterations in density or function of the L-type calcium channel have been implicated in a variety of cardiovascular diseases, including atrial fibrillation, heart failure, and ischemic heart disease (Yue et al. 1997; Van Wagoner et al. 1999; Balke and Shorofsky 1998; Timothy and He 2002; Aggarwal and Boyden 1995; Mukherjee and Spinale 1998). Accordingly, there may be a relationship between cardiovascular diseases and the pathophysiological changes in Ca2+ homeostasis regulated by the L-type calcium channel. Furthermore, several studies have shown that deregulation of Ca2+ through the L-type calcium channel plays a crucial role in the pathogenesis of cell death. Nevertheless, whether the L-type calcium channel is involved in cardiomyocyte damage induced by restraint stress and to what degree L-type calcium channel abundance and function are affected by stress remain largely unknown. Recently, we found that acute restraint stress could cause enhancement of the L-type calcium current (ICa-L), which correlated with up-regulation of the activation characters of the calcium channel. This reversible change results in transient calcium overload, then triggers apoptosis and eventually leads to cardiomyocytes injury (Xu et al. 2003). However, it is uncertain how changes in L-type calcium channels are regulated by chronic stress.
Against this background, the aims of this study were to observe the change of ICa-L in rat ventricular myocytes under chronic restraint stress using the whole-cell patch-clamp technique and to explore further its modulation mechanisms. Our findings suggest that chronic restraint stress could also enhance ICa-L, but that the change in ICa-L density might be mainly dependent on the abundance of the L-type calcium channel α1c subunit expression, which is different from the regulation mechanism of acute restraint stress.
Materials and methods
Experimental animal model of restraint stress
The animal model of restraint stress was established according to the method of Galea et al. (1997), with slight modifications. Adult male Wistar rats weighing 200–250 g were divided randomly into control and stress groups. All rats were housed in a pathogen-free environment at room temperature (RT; 22–25°C) and maintained on rat chow and tap water ad libitum before restraint stress. Individual rats in the stressed group were placed in a specially built size-manipulable cabin for 6 h/day for 21 consecutive days, and control rats were not disturbed during the 21-day period. Rats were killed 24 h after the last day of restraint.
Ventricular myocytes isolation
A single ventricular myocyte from the left ventricle of adult rat heart was enzymatically isolated by langendorff retrograde perfusion of the aorta (Isenberg and Klockner 1982). In brief, hearts were quickly removed, immersed in Ca2+-free Tyrode’s solution (4°C), and retrogradely perfused for 5 min with Ca2+-free Tyrode’s solution equilibrated with 95% O2 and 5% CO2 at 37°C to remove any excess blood in the vessels. Thereafter, the perfusate was switched to the “enzyme medium” of low Ca2+ (25 μmol/l) Tyrode’s solution containing collagenase type Ι (0.33 mg/ml, Sigma, St. Louis, MO, USA). After perfusion with the enzyme medium for 10–15 min, the hearts were perfused with the Kraft–Brühe (KB) medium for 5 min. Subsequently, the ventricles were cut out and chopped with scissors. The cells were released from the chunks by mechanical agitation and then separated by passing through a 200-μm mesh net into the KB medium. The cells were stored in KB medium for at least 1 h at 4°C before the experiments. This 1 h preincubation of the myocytes in KB medium results in a greater yield of Ca-tolerant cells. The ventricular myocytes measured 80–90 μm in length and 20–30 μm in diameter. Only rod-shaped cells with a regular striation pattern were selected for electrophysiological studies.
Measurement of the L-type calcium channel current
The whole-cell patch-clamp technique has made it possible to measure ionic fluxes across the cell membrane and has been particularly instrumental in measuring the magnitude and kinetics of ICa-L (Marty and Neher 1995).
Myocytes were placed in a chamber mounted on an inverted microscope (IX70, Olympus Inc., Tokyo, Japan) and continuously superfused with extracellular solution (1–2 ml/min). Glass microelectrodes (2–3 μm diameter) were pulled with a horizontal puller (P-87, Sutter Instrument, Novato, CA, USA), yielding a tip resistance of 3–5 MΩ when filled with pipette solution. After gigaseal was formed and the patch ruptured, an Axopatch-200B patch clamp amplifier (Axon Instruments, Union City, CA, USA) was used for voltage clamping, and ICa-L was obtained by voltage clamp steps of 200-ms duration from a −90 mV holding potential to test potentials between −80 and +40 mV. During current measurements, cell capacitance and series resistance were compensated (~80%), and pCLAMP 8 software package (Axon) were used for data acquisition and analysis. Current densities were determined by dividing current amplitudes by cell capacitance. All electrophysiology experiments were performed at physiologic temperatures (35–37°C).
RT-PCR and Northern blot analysis
Voltage-gated calcium channels are multimeric protein complexes consisting of at least four subunits. The major component of the channel is the pore-forming α1 subunit, which contains the binding site for calcium channel blockers, the voltage-sensor, and the selectivity filter. Subunit α1c is the primary isoform found in the heart and gives rise to high-voltage-activated ICa-L (Catterall 2000; Moosmang et al. 2005).
To determine the α1c subunit of L-type calcium channel expression levels, total RNA was extracted from ventricular myocardium by using Trizol reagent (Invitrogen, San Diego, CA, USA) and then analyzed by a reverse-transcription polymerase chain reaction (RT-PCR) and Northern blot method. RT-PCR (one step RNA PCR kit, TaKaRa, Tokyo, Japan) was performed with a primer specific for the cardiac L-type calcium channel subunit α1c (forward: 5′-CTACAACCAGGAGGGCATAAT-3′, reverse: 5′-TTGAAGCGGAAGCGGTA-3′) with the myocardial RNA as a template. The RT-PCR products were analyzed by electrophoresis on 1% agarose gels.
In a Northern blot assay, an aliquot of total RNA (10 μg) sample was separated on a 1% agarose gel containing formaldehyde and transferred to a nylon membrane by the capillary blotting. Hybridization was done using the RT-PCR products that had been radiolabeled with [α-32p]-dCTP by random priming (random primer DNA labeling kit, TaKaRa). Autoradiograph of a hybridization blot was visualized on X-ray film, and its density was quantified using an image software package (ImageMaster VDS, Amersham Pharmacia Biotech, Uppsala, Sweden). The β-actin signals were used as internal controls.
Western blot analysis of L-type calcium channel protein expression
To isolate proteins, ventricular myocardium tissues were homogenized in radioimmunoprecipitation (RIPA) lysis buffer with a protease-inhibitor and centrifuged at 4°C. Protein concentrations were measured by Bradford assay with bovine serum albumin (BSA) as a standard. Proteins were denatured in sodium dodecyl phosphate (SDS) loading buffer, electrophoresed on 8% SDS-polyacrylamide gels using a mini-Protean cell (Bio-Rad, Hercules, CA, USA) and then transferred to polyvinylidine difluoride membrane (Millipore, Bedford, MA, USA) using a Trans-Blot SD cell (Bio-Rad). For immunodetection, membranes were first incubated with primary antibody (C 4980, anti-cardiac α1c subunit, 1:200, Sigma) overnight at 4°C and with secondary antibody (horseradish-peroxidase-conjugated goat anti-rabbit IgG, Zhongshan, Beijing, China) for 2 h at RT. Immunoreactive bands were visualized on X-ray film using the chemiluminescence method. Band densities were quantified by densitometry, standardized to α-tubulin, and normalized to the control sample on each gel.
Solutions
For myocyte isolation, we used the following solutions: Ca2+-free Tyrode’s solution containing (in mmol/l) NaCl 116, KCl 5.4, NaH2PO4 1.4, NaHCO3 15, MgSO4 1, glucose 15, and 4-2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES) 5 (pH 7.4 adjusted with NaOH); KB medium containing (in mmol/l) l-glutamic acid 70, KCl 25, taurine 20, KH2PO4 10, MgCl2 3, HEPES 10, glucose 10, and ethylene glycol tetraacetic acid (EGTA) 0.5 (pH 7.4 adjusted with KOH).
The extracellular solution for ICa-L studies was composed of (in mmol/l): choline chloride 120, CsCl 4, CaCl2 1.8, MgCl2 2, HEPES 10 and glucose 10 (pH 7.4 adjusted with CsOH).
The pipette solution for ICa-L recording contained the following (in mmol/l): CsCl 120, MgCl2 2, HEPES 10, EGTA 10, K2ATP 5, and tetraethylammonium ions (TEA) 10 (pH 7.3 adjusted with CsOH).
Statistics
All data are given as mean ± standard deviation (SD). Statistical significance was performed by Student’s t test, and differences were considered significant at P < 0.05.
Results
Detection of the L-type calcium channel current in rat ventricular myocytes
In cardiac myocytes, two types of Ca2+ have been identified: the T-type and the L-type calcium channels. The nomenclature of these channels is based upon their activation and current-carrying capacities. While the T-type calcium channel is characterized by transient openings and very low conductance, the L-type calcium channel is characterized by a long-lasting opening, high conductance, and activation at larger membrane depolarizations. The L-type calcium channel is activated at −40 to −30 mV, and a peak influx of Ca2+ ions through the L-type calcium channel has been demonstrated to occur at membrane potentials of 0 to +10 mV (Bean 1985; Bers 1991; Catterall 1995). Thus, a major determinant of the action potential plateau of the cardiac myocyte is the influx of Ca2+ ions through the L-type calcium channel.
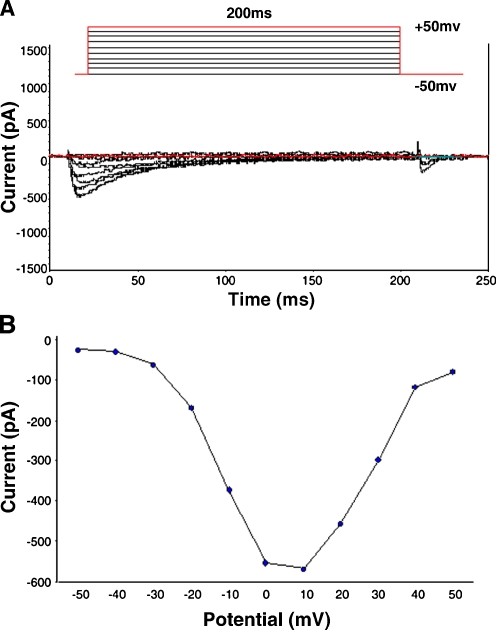
The calcium current was activated by a depolarizing pulse from a holding potential of −50 to +50 mV at 10 mV step-voltage. This inward current could be completely inhibited (90%) by 5 μmol/l nifedipine, a specific L-type calcium channel blocker. The basic characteristics indicated that the major current present in rat ventricular myocytes was ICa-L (data not shown). Figure 1 shows representative results. The peak of ICa-L occurred at membrane potentials of 0 to +10 mV under control conditions and threshold potentials at −30 mV.
Fig. 1.
Properties of basal L-type calcium current (ICa-L) in rat ventricular myocytes. A Representative traces of whole-cell ICa-L. Superimposed ICa-L was recorded at depolarizing pulse from a holding potential of −50 to +50 mV at 10 mV step-voltage with whole-all voltage-clamp method. The duration of the applied test voltages was 200 ms. Pulse protocol was shown on the top. B Current versus voltage relationship for mean peak ICa-L. The peak of ICa-L occurred at membrane potentials of 0 to +10 mV under control conditions and threshold potentials at −30 mV
ICa-L up-regulation by chronic restraint stress
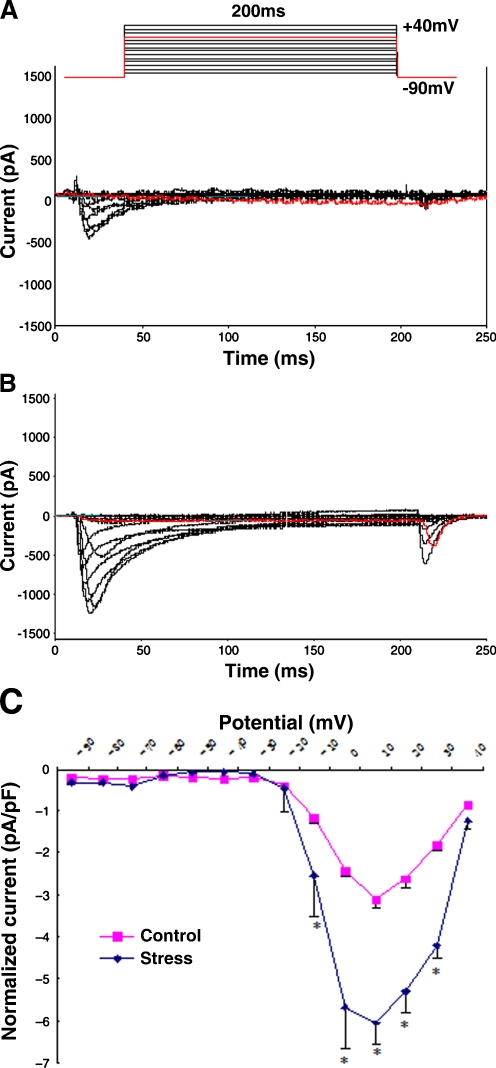
ICa-L were elicited from a holding potential of −90 mV to test potentials ranging from −80 to +40 mV in 10-mV increment steps for 200 ms using the whole-cell patch clamp technique, and the effects of stress were investigated. Representative current traces in ventricular myocytes isolated from control and chronic restraint stress rats are shown in Fig. 2. The amplitude of peak ICa-L at all test potential was higher in the cells isolated from stress (B) than from the control (A). The peak of ICa-L recorded at each step of the voltage pulse protocol was plotted as a function of the voltage step to construct the current–voltage relationship. To account for cell size, measured ICa-L is commonly normalized to the membrane capacitance, an index of cell surface area. As illustrated in Fig. 2C, in both the control and the stress groups, ICa-L were activated by depolarization above −25 mV approximately and at peak current at around +10 mV. Moreover, ICa-L density was markedly increased in ventricular myocytes obtained from stress (−6.04 ± 0.98 pA/pF) compared to those from the control (−3.13 ± 0.02 pA/pF, n = 6, P < 0.05). These results suggest that chronic stress could enhance ICa-L.
Fig. 2.
Alterations of ICa-L in rat ventricular myocytes under chronic restraint stress. A, B. Representative ICa-L traces for control and chronic stress. Superimposed ICa-L were elicited from a holding potential of −90 mV to test potential ranging from −80 to +40 mV in 10 mV increment steps for 200 ms using the whole-cell patch clamp technique. The voltage-clamp protocol is shown schematically above the traces. The amplitude of peak ICa-L at all test potential was larger in the ventricular myocytes isolated from chronic stress rats (B) than control (A). C Current density versus voltage relationship of mean peak ICa-L for control and chronic stress cells. The peak ICa-L recorded at each step of the voltage pulse protocol is plotted as a function of the voltage step to construct the current–voltage relationship. To account for cell size, current density (pA/pF) was determined by dividing current amplitude by cell capacitance (an index of cell surface area). Current density increased markedly in ventricular myocytes obtained from chronic stress (−6.04 ± 0.98 pA/pF, n = 6) rats compared to control (−3.13 ± 0.02 pA/pF, n = 6, P < 0.05)
Meanwhile, the effects of stress on the steady-state activation and inactivation characters of the L-type calcium channel in ventricular myocytes were observed as described before. However, there was no significant difference in the activation and inactivation characters of the L-type calcium channel between chronic restraint stress and control (n = 6; data not shown). That indicates that the molecular mechanism underlying the chronic stress-induced ICa-L increase may be different from that of acute stress.
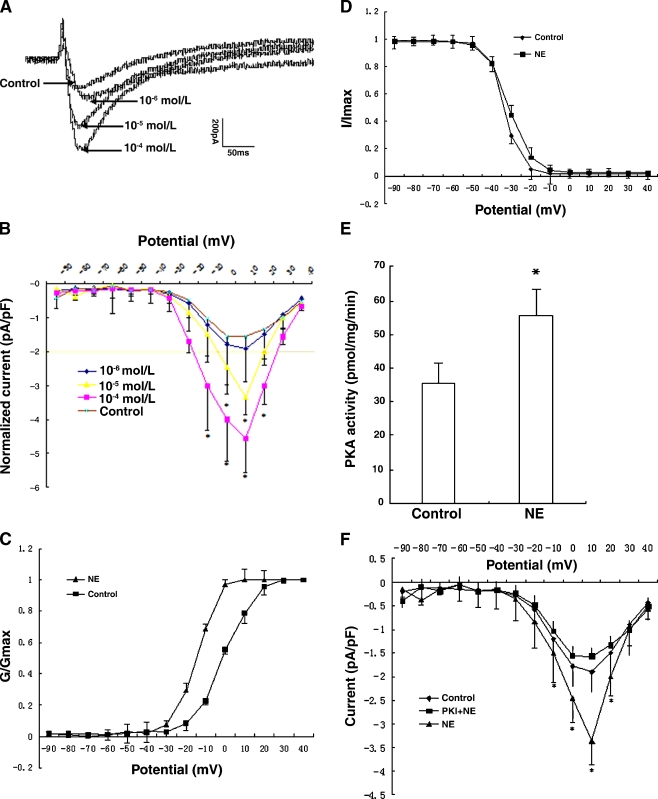
In our recent findings (Fig. 3), ICa-L were significantly increased (P < 0.05) under acute stress (exposure of myocytes to 10−6, 10−5, 10−4 mol/l NE) in a dose-dependent manner. In addition, after treatment with 10−4 mol/l NE, the steady-state activation curve of the L-type calcium channel showed that the half-maximal activation voltage (V1/2) changed from −0.69 ± 0.36 to −14.59 ± 0.24 mV (P < 0.05, n = 8), with a shift in the activation curve to more negative potentials. The alteration of a steady-state inactivation curve was not detected, indicating that the L-type calcium channels of ventricular myocytes were inclined to be activated under acute stress. The results demonstrated that up-regulation of ICa-L is associated with an alteration in phosphorylation-dependent channel activation. Furthermore, activity of protein kinase A (PKA) increased markedly after acute stress, and ICa-L was inhibited by protein kinase inhibitor (PKI), suggesting that acute stress-induced ICa-L increase involves activation by PKA.
Fig. 3.
Effect of acute stress on the L-type calcium channels in rat ventricular myocytes. A Peak ICa-L traces for control and NE (norepinephrine)-activated. Peak ICa-L was increased under acute stress (i.e., exposure of ventricular myocytes to 10−6, 10−5 and 10−4 mol/l NE for 10 min) in a dose-dependent manner. B Current density versus voltage relationship of mean peak ICa-L for control and NE. Peak ICa-L density was significantly increased in ventricular myocytes in response to 10−4 and 10−5 mol/l NE (−4.56 ± 0.95 and −3.39 ± 0.87 pA/pF, n = 8) compared to control (−1.55 ± 0.43 pA/pF, n = 8, P < 0.05). C, D. Activation and inactivation curves of the L-type calcium channel. For activation, conductance was determined by dividing the peak current amplitude at each potential by the driving force for Ca2+. The continuous lines represent the best fits of Boltzmann equations to the data. The half-maximal voltage required for activation was −14.59 ± 0.24 and −0.69 ± 0.36 mV in ventricular myocytes with 10−4 mol/l NE treatment and control (n = 8, P < 0.05), respectively, and there is a shift in the activation curve to more negative potentials (C). While the two steady-state inactivation curves were nearly identical, the half-maximal inactivation voltage was −33.57 ± 0.31 mV (control) and −31 ± 0.48 mV (10−4 mol/l NE; n = 7, P > 0.05), respectively (D). It indicates that the L-type calcium channel in rat ventricular myocytes was inclined to be activated under acute stress. E, F Regulation of the L-type calcium channel by protein kinase A (PKA) in ventricular myocytes. PKA activity was markedly increased after10−4 mol/l NE treatment, from 35.50 ± 6.34 (control) to 55.59 ± 7.51 pmol/mg/min (n = 8, P < 0.05) (E). Then, ICa-L was measured in the presence of protein kinase inhibitor (PKI), in contrast to NE group, ICa-L was significantly decreased (F).It suggests that acute stress-induced ICa-L increase involves activation by PKA
Expression of the L-type calcium channel α1c subunit enhancement by chronic stress
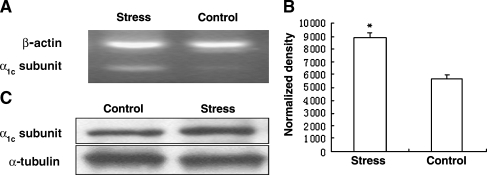
The purpose of this experiment was to determine whether or not expression levels of L-type calcium channel α1c subunit were regulated by chronic stress and whether or not this change was related to the up-regulation of ICa-L in ventricular myocytes under chronic stress. Semi-quantitative RT-PCR analysis was first used to verify the hypothesis. As shown in Fig. 4A, the α1c subunit of the L-type calcium channel was expressed in both groups and had a higher expression level in the ventricular myocardium from rats under chronic stress than those in the control. In addition, Northern blot analysis was performed to evaluate the effect of chronic stress on the mRNA levels. Consistent with the finding obtained by RT-PCR, the result from Northern blot showed that the α1c subunit mRNA of the L-type calcium channel in chronic stress was more abundant and increased markedly to approximately 1.6-fold compared to the control (P < 0.05, n = 4; Fig. 4B). Furthermore, immunoblot analysis also revealed that the amount of α1c subunit protein was increased in the heart of stressed rats, about 17% more than control (Fig. 4C). Our results, together with a previous work on L-type calcium channel, suggest that chronic stress could lead to up-regulation of α1c subunit expression, and it may be a major molecular mechanism underlying the ICa-L increased induced by chronic stress.
Fig. 4.
Change in expression of L-type calcium channel α1c subunit in rat ventricular myocardium. The cardiac L-type calcium channel is a membranous multimer consisting of the pore-forming α1c subunit and the regulatory α2/δ and β subunits. The α1c subunit contains voltage sensors and receptor sites for the different classes of calcium channel antagonists/agonists, and determines the basic electrophysiological properties. A Semi-quantitative RT-PCR analysis shown the α1c subunit of L-type calcium channel was expressed in both control and chronic stress groups and that it had a higher expression level in ventricular myocardium from chronic stress rat than control. B The results from Northern blot showed the α1c subunit mRNA of the L-type calcium channel in chronic stress was more abundant and increased markedly to approximate 1.6-fold compared to control (P < 0.05, n = 4). β-actin was used as an internal control. C Western blot analysis also revealed that the amount of α1c subunit protein was increased in the heart of stressed rats, about 17% more than control. α-tubulin was used as the loading control. It suggests that chronic stress could lead to up-regulation of α1c subunit expression
Discussion
The voltage-gated L-type calcium channel plays a pivotal role in the regulation of a wide range of cellular processes, including membrane excitability, Ca2+ homeostasis, protein phosphorylation, and gene regulation (Brosenitsch et al. 1998). In the heart, excitation–contraction coupling depends on the L-type calcium channel. The cardiac L-type calcium channel is a membranous multimer consisting of the pore-forming α1c subunit and the regulatory α2/δ and β subunits. The α1c subunit contains voltage sensor and receptor sites for the different classes of calcium channel antagonists/agonists and determines the basic electrophysiological properties (Schultz et al. 1993).
In the present study, we have shown that chronic stress enhanced ICa-L density and increased ICa-L that was not accompanied by alteration of the characteristics of activation and inactivation, but corresponded with up-regulation in the mRNA and protein levels of the L-type calcium channel α1c subunit. Past studies have demonstrated that the relation between the number of L-type calcium channels and whole-cell ICa-L may be expressed as: I = NT × Pf × i × Po, where I is the whole-cell current, NT is the total number of channels, Pf is the probability that the available channels are functional, i is the total current that any single channel can carry, and Po is the probability that the functional channel is open (Bers 1991; Klockner and Isenberg 1994; Campbell and Strauss 1995). Since ICa-L density is obtained by dividing ICa-L amplitude by membrane capacitance (an electrical index of membrane surface area), then, by definition, a significant correlation must exist between L-type calcium channel abundance and ICa-L density. Thus, conditions that change L-type calcium channel abundance could influence whole-cell ICa-L density. As our findings have shown that the characteristics of activation and inactivation were not affected by chronic restraint stress, it may be speculated that the expression alteration of the L-type calcium channel in ventricular myocytes underlies the up-regulation of ICa-L. RT-PCR, Northern blot, and Western blot assays confirmed the hypothesis that the abundance of mRNA and protein of the L-type calcium channel α1c subunit in the ventricle was increased significantly in the chronically stressed rat. Considering these results, the change in ICa-L density might be mainly dependent on the abundance of the L-type calcium channel under chronic stress, i.e., the expression regulation. This modulation change is irreversible, and is different from the change induced by acute stress. Numerous studies have demonstrated that functional regulation of the calcium channel relies on phosphorylation processes, such as PKA and PKC, which all affect ICa-L. In the heart, phosphorylation of ICa-L is counteracted by type 1 and type 2A phosphatases. Thus, the actual amplitude of basal ICa-L is determined by the balanced activity of kinases and phosphatases (Kamp and Hell 2000; Herzig and Neumann 2000; Christ et al. 2004). This confirms the findings in our previous research, which found that acute stress only caused cardiac dysfunction, whereas chronic stress may lead to an organic pathological change of the heart.
Calcium ions are central to multiple signal transduction pathways that accomplish a variety of biological functions. The spatial and temporal regulation of intracellular calcium ([Ca2+]i) serves as a modulator of pathways involved in fertilization, proliferation, and development (Berridge et al. 2000). However, it is apparent that modulation of [Ca2+]i plays a very important role in the pathogenesis of cell injury and cell death (Trump and Berezesky 1995). Several studies have shown that increased Ca2+ influx through the L-type calcium channel has been implicated in the apoptosis of cardiomyocytes induced by ischemia/reperfusion, catecholamines, and angiotensin II (Gao et al. 2001; Communal et al. 1998; Goldenberg et al. 2001). Moreover, a higher myocyte apoptotic rate was observed in the intact heart of a line of transgenic mice overexpressing the α1c subunit (Muth et al. 2001), suggesting a role for persistent increases in Ca2+ influx in apoptosis in vivo. The Ca2+ homeostasis in the cell is maintained by the balance among the calcium channel, the endoplasmic reticulum (ER), and the mitochondria. Any deregulation among them can cause disruption of the Ca2+ equilibrium, such as Ca2+ overload, that is harmful and may trigger apoptosis (Demaurex and Distelhorst 2003; Hajnoczky et al. 2000). This was also confirmed in our previous study, but the interaction details between Ca2+ ions and apoptosis needed to be clarified further. A recent study reported that the transfer of Ca2+ from the ER to the mitochondria is required for initiation of programmed cell death by some (Scorrano et al. 2003).
In conclusion, our results show that chronic restraint stress could enhance ICa-L, and the up-regulated expression of the L-type calcium channel α1c subunit might contribute to altered calcium handling in stress-induced cardiomyocyte injury, which is different from the regulation mechanism of acute restraint stress that mostly related to activation of the PKA signal transduction pathway. The precise regulation of the Ca2+ influx in response to various physiological situations is further controlled by several regulatory mechanisms, working at different levels, including channel expression, localization, or activity, via additional interactions with modulatory proteins. There are some limitations in the present study, such as the relationship between the expression level of all the subunits and the electrophysiological function of the whole channel has not yet been clarified. Therefore, a combined investigation of the mRNA levels, protein levels, histological distribution, and electrophysiological functions is required. Although limited for these reasons, the present result provides a new understanding of the mechanism of cardiomyocyte injury induced by stress and may help in the prevention and cure of cardiovascular diseases.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NNSFC; 30430590) and the National High Technology Research Development Program of China (2006AA02Z4C8).
Abbreviations
- ICa-L
L-type calcium current
- RT-PCR
reverse-transcription polymerase chain reaction
- PKA/C
protein kinase A /C
- [Ca2+]i
intracellular calcium
- ER
endoplasmic reticulum
- NE
norepinephrine
Footnotes
Y. Zhao and J. Xu contributed equally to this work.
References
- Aggarwal R, Boyden PA. Diminished calcium and barium currents in myocytes surviving in the epicardial border zone of the 5-day infracted canine heart. Circ Res. 1995;77:1180–1191. doi: 10.1161/01.res.77.6.1180. [DOI] [PubMed] [Google Scholar]
- Balke CW, Shorofsky SR. Alterations in calcium handling in cardiac hypertrophy and heart failure. Cardiovasc Res. 1998;37:290–299. doi: 10.1016/S0008-6363(97)00272-1. [DOI] [PubMed] [Google Scholar]
- Bean BP. Two kinds of calcium channels in canine atrial cells. J Gen Physiol. 1985;86:1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bers DM. Ca influx via sarcolemmal Ca channels. In: Bers DM, editor. Excitation-contraction coupling and cardiac contractile force. Norwell, MA: Kluwer; 1991. pp. 49–70. [Google Scholar]
- Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch TA, Salgado Commissariat D, Kunze DL, Katz DM. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. Neurosci. 1998;18:1047–1055. doi: 10.1523/JNEUROSCI.18-03-01047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DL, Strauss HC. Regulation of calcium channels in the heart. Adv Second Messenger Phosphoprot Res. 1995;30:25–88. doi: 10.1016/s1040-7952(05)80004-7. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–501. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Christ T, Boknik P, Wöhrl S, et al. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- Das DK, Engelman RM, Maulik N (1999) Oxygen free radical signaling in ischemic preconditioning. In: Das DK (ed) Heart in stress . Ann NY Acad Sci 874:49–65 [DOI] [PubMed]
- Demaurex N, Distelhorst C. Apoptosis—the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Young PR. Apoptosis in cardiac diseases: stress- and mitogen-activated signaling pathways. Cardiovasc Res. 2000;45:560–569. doi: 10.1016/S0008-6363(99)00372-7. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Mcewen BS, Tanapat P, et al. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neurosci. 1997;81:689–697. doi: 10.1016/S0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gao F, Gong B, Christopher TA, Lopez BL, Karasawa A, Ma XL. Anti-apoptotic effect of benidipine, a long-lasting vasodilating calcium antagonist, in ischaemic/reperfused myocardial cells. Br J Pharmacol. 2001;132:869–878. doi: 10.1038/sj.bjp.0703881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg I, Grossman E, Jacobson KA, Shneyvays V, Shainberg A. Angiotensin II-induced apoptosis in rat cardiomyocyte culture: a possible role of AT1 and AT2 receptors. J Hypertens. 2001;19:1681–1689. doi: 10.1097/00004872-200109000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Madesh M, Pacher P. Control of apoptosis by IP3 and ryanodine receptor driven calcium signals. Cell Calcium. 2000;28:349–363. doi: 10.1054/ceca.2000.0169. [DOI] [PubMed] [Google Scholar]
- Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a “KB” medium. Pflugers Arch. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kamp TJ, Hell JW. Regulation of L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;22:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- Klockner U, Isenberg G. Intracellular pH Modulates the Availability of Vascular L-type Ca2+ Channels. J Gen Physiol. 1994;103:647–663. doi: 10.1085/jgp.103.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A, Neher E. Tight-seal whole-cell recording. In: Sakmann B, Neher E, editors. Single-channel recording. 2. New York: Plenum; 1995. pp. 31–52. [Google Scholar]
- Moosmang S, Lenhardt P, Haider N, Hofmann F, Wegener JW. Mouse models to study L-type calcium channel function. Pharmacol Ther. 2005;106:347–355. doi: 10.1016/j.pharmthera.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Spinale FG. L-type calcium channel abundance and function with cardiac hypertrophy and failure: A review. J Mol Cell Cardiol. 1998;30:1899–1916. doi: 10.1006/jmcc.1998.0755. [DOI] [PubMed] [Google Scholar]
- Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca2+-dependent transgenic model of cardiac hypertrophy: A role for protein kinase Calpha. Circulation. 2001;103:140–147. doi: 10.1161/01.cir.103.1.140. [DOI] [PubMed] [Google Scholar]
- Schultz D, Mikala G, Yatani A, et al. Cloning, chromosomal localization, and functional expression of the 1 subunit of the L-type voltage-dependent calcium channel from normal human heart. Proc Natl Acad Sci USA. 1993;90:6228–6232. doi: 10.1073/pnas.90.13.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AR, Gerin W, Davidson KW, Picking TG, Brosschot JF, Thayer JF, Christenfeld N, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosom Med. 2003;65:22–35. doi: 10.1097/01.PSY.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–159. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Timothy JK, He JQ. L-type Ca2+ channels gaining respect in heart failure. Cardiovasc Res. 2002;91:451–453. doi: 10.1161/01.res.0000035346.21625.4a. [DOI] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB. 1995;9:219–228. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]
- Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- Xu J, Ma Q, Duan HF, Qian LJ. Effects of stress on L-type calcium channels of rat ventricular myocytes. Chin J Applied Physiol. 2003;19:216–219. [PubMed] [Google Scholar]
- Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang WY, Qian LJ. Hsp70 may protect cardiomyocytes from stress -induced injury by inhibiting Fas-mediated apoptosis. Cell Stress Chaperones. 2007;12:83–95. doi: 10.1379/CSC-231R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]