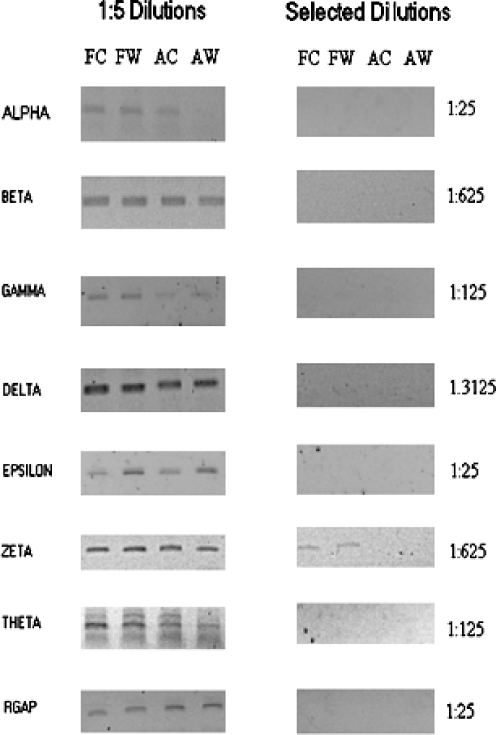
Fig. 1.
Serial-limiting dilution assay of CCT subunits. The wounding protocol for our New Zealand white rabbits and RNA isolation/storage from fetal and adult wound tissues was done as previously described (Darden et al. 2000; Kathju et al. 2006). All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. RNA levels were assayed for seven chaperonin subunits using limiting dilution reverse transcription-polymerase chain reaction (RT-PCR) as previously described (Darden et al. 2000). Limiting dilution RT-PCR assays were performed on 200 ng of pooled total RNA from fetal control (FC), fetal wound (FW), adult control (AC), and adult wound (AW) samples; each pool was comprised of equivalent amounts from at least four separate biological samples; assays were performed in triplicate. Undiluted samples were reverse transcribed and subjected to 35 cycles of PCR (using subunit appropriate primers) and compared to serial fivefold dilutions (1:5, 1:25,1:125, 1:625; 1:3125) tested at the same time in the same conditions. Negative controls, that is, PCR reactions without an initial reverse transcriptase step, were also included to ensure that the amplimers seen are derived from expressed mRNA and not contaminating chromosomal DNA. Rabbit GAPDH was used as an invariant internal control. The left panel displays results of 1:5 dilutions for all subunits; the alpha subunit already has lost a band in the AW lane, indicating it is less abundant in adult wound. The right panel shows the result, in each case, of the first dilution at which a band becomes undetectable (indicated at right). Differences in the end dilution points reflect technical variation between subunit assays and are not themselves indicative of differential subunit expression. Rabbit GAPDH (RGAP) is invariant across tissue types