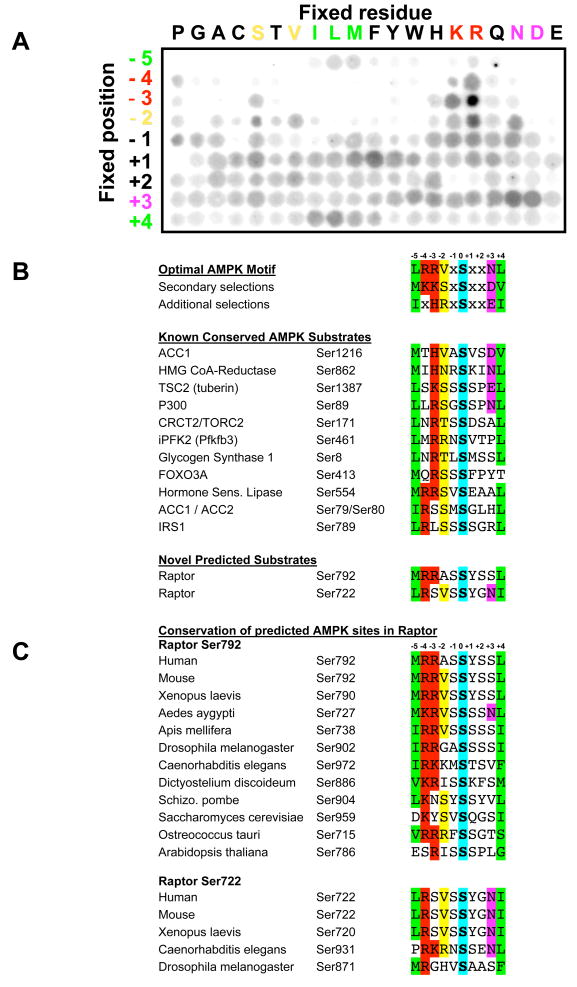
Figure 1. Peptide library profiling the optimal substrate motif for AMPK and comparison with known and candidate in vivo phosphorylation sites.
A. A spatially arrayed PSPL was subjected to in vitro phosphorylation with active AMPKα1 and radiolabelled ATP. Each peptide contained one residue fixed at one of nine positions relative to the centrally fixed phospho-acceptor (an equal mix of serine and threonine). Aliquots of each reaction were spotted onto avidin membrane, which was washed, dried and exposed to a phosphor storage screen, providing the array of spots shown in the figure. AMPK displayed strong selectivity at the −5, −4, −3, −2, +3, and +4 positions.
B. The optimal and secondary selections taken from triplicate analyses as in A are displayed. AMPK phosphorylation sites in the best established in vivo substrates of AMPK conform to the substrate motif derived from the peptide library data. All substrates shown were isolated in bioinformatics searches for proteins containing a conserved AMPK phosphorylation motif. These same searches yielded two novel predicted AMPK sites in raptor.
C. The predicted AMPK sites in raptor are highly conserved across evolution.