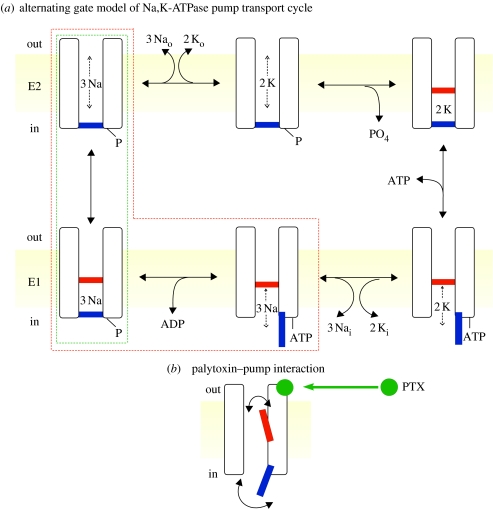
Figure 2.
(a) Alternating-gate model of the Post–Albers (Post et al. 1965; Albers 1967) transport cycle of the Na+,K+-ATPase pump represented in cartoon form as an ion channel with two gates, an extracellular-side (labelled out) gate (red) and cytoplasmic-side (in) gate (blue), that open alternately, but are never simultaneously open. Also indicated are E2 (upper row; extracellular-side gate may open) and E1 (lower row; cytoplasmic-side gate may open) states. Occluded states, with both gates shut, follow binding of two external K+ (top right) and of three internal Na+ and subsequent phosphorylation (bottom left). ATP acts with low affinity to speed up the opening of the cytoplasmic-side gate and concomitant K+ deocclusion, and acts with high affinity to phosphorylate the pump. The states enclosed by the red dashed box are occupied in the presence of ATP and Na+ ions, but in the absence of K+ ions. The states within the green dashed box are occupied in the presence of ATP and Na+ ions, but in the absence of ADP and K+ ions, and yield voltage-jump-induced charge movements, associated with deocclusion and release to the exterior of the three transported Na+ ions (Holmgren et al. 2000). (b) Cartoon of channel-like Na+,K+ pump with bound palytoxin (PTX; green ball), which allows the pump's two gates sometimes to be open at the same time. (Modified from Artigas & Gadsby 2003.)