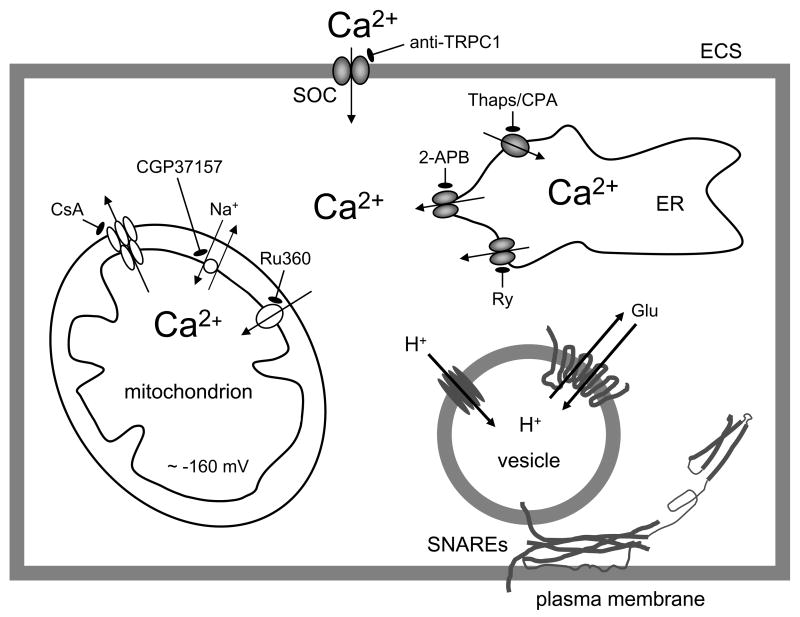
Figure 1.
Tripartite Ca2+cyt handling in Ca2+- dependent vesicular glutamate release from astrocytes. A drawing of sources of Ca2+ for [Ca2+]cyt increase. [Ca2+]cyt can be affected by the endoplasmic reticulum (ER), store-operated Ca2+ (SOC) entry and mitochondria. Ca2+cyt accumulation could be caused by the entry of Ca2+ from the internal stores of the ER that possess IP3 and ryanodine receptors. The pre-incubation of astrocytes with ryanodine (Ry) can block Ca2+ release from ryanodine/caffeine-sensitve ER stores, while diphenylboric acid 2-aminoethyl ester (2-APB), an IP3R antagonist, reduces Ca2+ release from an IP3-senistive stores. Thapsigargin (Thaps) and cyclopiazonic acid (CPA), blockers of store specific Ca2+-ATPase, prevent filling of these stores by Ca2+ and, thus, deplete stores of this ion. Ultimately, the (re)filling of ER Ca2+ stores requires Ca2+ entry from the extracellular space (ECS) through SOC channels, in particular those containing canonical type 1 transient receptor potential (TRPC1) protein. A specific TRPC1 antibody blocks TRPC1 containing SOC channels and reduces Ca2+ entry to the cytosol. A negative membrane potential (about −160 mV) exists across the inner mitochondrial membrane. Mitochondrial Ca2+ uptake is mediated by the uniporter as the electropotential gradient drives Ca2+ into the matrix. Ru360 blocks Ca2+ influx through the uniporter. Free Ca2+ exits the mitochondrial matrix through the Na+/Ca2+ exchanger, and this process can be blocked by CGP37157. Ca2+ accumulation in mitochondria is increased by cyclosporin A (CsA) via preventing formation of the mitochondrial permeability transition pore, which releases Ca2+ and other components of the mitochondrial matrix at high Ca2+ loads. Increase of Ca2+cyt is sufficient and necessary to cause vesicular fusions and release of glutamate, which requires action by proteins of the ternary SNARE complex and their associated proteins (not shown). Additionally, vesicles possess the vacuolar type H+-ATPase which creates the proton gradient used by vesicular glutamate transporters to fill these organelles with glutamate (Glu). Drawing is not to scale.