Abstract
Background
Studies in mouse, Xenopus and chicken have shown that Otx2 and Gbx2 expression domains are fundamental for positioning the midbrain-hindbrain boundary (MHB) organizer. Of the two zebrafish gbx genes, gbx1 is a likely candidate to participate in this event because its early expression is similar to that reported for Gbx2 in other species. Zebrafish gbx2, on the other hand, acts relatively late at the MHB. To investigate the function of zebrafish gbx1 within the early neural plate, we used a combination of gain- and loss-of-function experiments.
Results
We found that ectopic gbx1 expression in the anterior neural plate reduces forebrain and midbrain, represses otx2 expression and repositions the MHB to a more anterior position at the new gbx1/otx2 border. In the case of gbx1 loss-of-function, the initially robust otx2 domain shifts slightly posterior at a given stage (70% epiboly), as does MHB marker expression. We further found that ectopic juxtaposition of otx2 and gbx1 leads to ectopic activation of MHB markers fgf8, pax2.1 and eng2. This indicates that, in zebrafish, an interaction between otx2 and gbx1 determines the site of MHB development. Our work also highlights a novel requirement for gbx1 in hindbrain development. Using cell-tracing experiments, gbx1 was found to cell-autonomously transform anterior neural tissue into posterior. Previous studies have shown that gbx1 is a target of Wnt8 graded activity in the early neural plate. Consistent with this, we show that gbx1 can partially restore hindbrain patterning in cases of Wnt8 loss-of-function. We propose that in addition to its role at the MHB, gbx1 acts at the transcriptional level to mediate Wnt8 posteriorizing signals that pattern the developing hindbrain.
Conclusion
Our results provide evidence that zebrafish gbx1 is involved in positioning the MHB in the early neural plate by refining the otx2 expression domain. In addition to its role in MHB formation, we have shown that gbx1 is a novel mediator of Wnt8 signaling during hindbrain patterning.
Background
Patterning of the vertebrate neural plate depends on the formation of local organizing centers that release effector molecules to control the regionalization of adjacent tissue. The first step in establishing an organizing center is proposed to be the specification of two distinct adjacent cell populations that subsequently undergo local cell-cell interactions to influence cell fate [1]. This model applies in part to the formation of the midbrain-hindbrain boundary (MHB) organizer, a source of signals that direct regional specification of both the midbrain and anterior hindbrain (reviewed in [2,3]). In mouse, the MHB arises from subdivision of the early neural plate into two domains that express distinct transcription factors: an anterior Otx2 expressing domain that encompasses both forebrain and midbrain primordia [4-6], and a posterior Gbx2 expressing domain that encompasses presumptive anterior hindbrain [7]. Intriguingly, gbx function during MHB formation in zebrafish does not appear to be carried out by gbx2. We have previously shown that gbx2 is expressed too late for such a role [8], and loss-of-function assays suggest that gbx2 functions in MHB maintenance, rather than formation [9]. Thus, it remains unknown what gene in zebrafish acts alongside otx2 to subdivide the neural plate early in MHB formation. Here we investigate a potential role for zebrafish gbx1, the other gbx homolog, during early neural patterning, and find that it has an important function during MHB formation as well as a novel role in the developing hindbrain.
Proper MHB organizer formation is defined by a cascade of genetic interactions that are marked by complex temporo-spatial patterns of gene expression. At the end of gastrulation, the transcription factors Pax2/5 and En1/2, and the secreted molecules Wnt1 and Fgf8 are expressed at the Otx2/Gbx2 interface (reviewed in: [2,10,11]). Studies in mice, chicken, and zebrafish indicate that three parallel signaling pathways, involving Pax2/pax2.1, Wnt1 and Fgf8, are activated independently at this interface [12,13] (reviewed in [10,14]). These three pathways become mutually dependent within a regulatory loop that is crucial for continued MHB development and its signaling activities during somitogenesis (reviewed in [2,10]). Otx2 or Gbx2 are necessary for correct antero-posterior (A-P) positioning of Fgf8 and Pax2 expression domains in mouse, although they are not required to initiate expression [7,15-19]. In Otx2 or Gbx2 loss-of-function mutants, this loss of positional information is reflected in the formation of large overlapping Fgf8 and Pax2 domains [7,15-19].
Mutual antagonism between Otx2 and Gbx2 has been shown to determine MHB position in mouse [17,20] and Xenopus [21,22]. Gbx2 misexpression in the caudal midbrain under the control of the Wnt1 promoter represses Otx2 expression and shifts the MHB organizer rostrally [17]; conversely, Otx2 misexpression in the rostral hindbrain under the En1 promoter represses Gbx2 expression and shifts the MHB posteriorly [20]. In both cases, Fgf8 expression is localized at the new Otx2/Gbx2 interface. Studies in chicken also have shown that ectopic juxtaposition of Gbx2 and Otx2 expression domains can induce MHB marker expression [23-25]. In sum, these experimental data suggest that negative interactions between Otx2 and Gbx2 generate a sharp boundary between their two expression domains and that the region where Otx2 and Gbx2 abut might demarcate the MHB primordium (reviewed in [10,11,26-28]).
Similar to Gbx2 expression in mouse [7,29], zebrafish gbx1 is expressed in the prospective hindbrain/spinal cord, adjacent to the otx2 domain, from the onset of gastrulation (70% epiboly) [8]. Additionally, all known players in the maintenance and/or organizing activity of the MHB (pax2.1/5, eng1/2, wnt1, fgf8) start to be expressed on either one or both sides of the gbx1/otx2 border. In contrast to zebrafish, mouse Gbx1 is never expressed at the MHB [30,31] and, as of yet, no functional data concerning Gbx1 are available.
In the present study, we set out to ascertain the function of gbx1 during early development in zebrafish. We show that ectopic gbx1 expression represses otx2 and shifts the MHB anteriorly to sit at the newly created gbx1/otx2 interface. Conversely, loss of gbx1 function results in a slight posterior expansion of the otx2 domain at 70% epiboly that is accompanied by a shift in MHB markers. These results provide evidence that, in zebrafish, gbx1 is playing an analogous role to Gbx2 in terrestrial vertebrates in positioning the MHB in the early neural plate by refining the otx2 expression domain.
Zebrafish gbx1 is also known to be expressed in the hindbrain territory during gastrulation [8], suggesting that it may have an additional function in hindbrain development. In agreement with this, we find that gbx1 gain- and loss-of-function affects the hindbrain territory as well as the MHB. Using cell tracing experiments we show that gbx1 induces posterior neural cell fate via cell-autonomous transformation of anterior neural tissue. Given that gbx1 is a target of the posteriorizing signal Wnt8 [32], and our finding that gbx1 overexpression rescues hindbrain loss in cases of Wnt8 loss-of-function, we conclude that in addition to its role in MHB formation, gbx1 is a novel mediator of Wnt8 signaling during hindbrain patterning.
Results
Ectopic gbx1 represses otx2 expression and expands the hindbrain
To study gbx1 function within the zebrafish early neural plate, we first injected synthetic gbx1 mRNA into one-cell stage embryos. The injected embryos showed an enlarged hindbrain and a strong reduction of anterior neural structures, lacking eyes, fore- and midbrain at 24 h (Figure 1A, B; see also [9]). Staining of early axon tracts with an anti-acetylated tubulin antibody revealed that this enlarged hindbrain was specified normally (Mauthner neuron and trigeminal ganglion are present; Figure 1C, D, arrowheads). Apart from the posteriorized neural phenotype, gbx1 overexpressing embryos also produced additional ear structures (Figure 1E, F, arrows).
Figure 1.
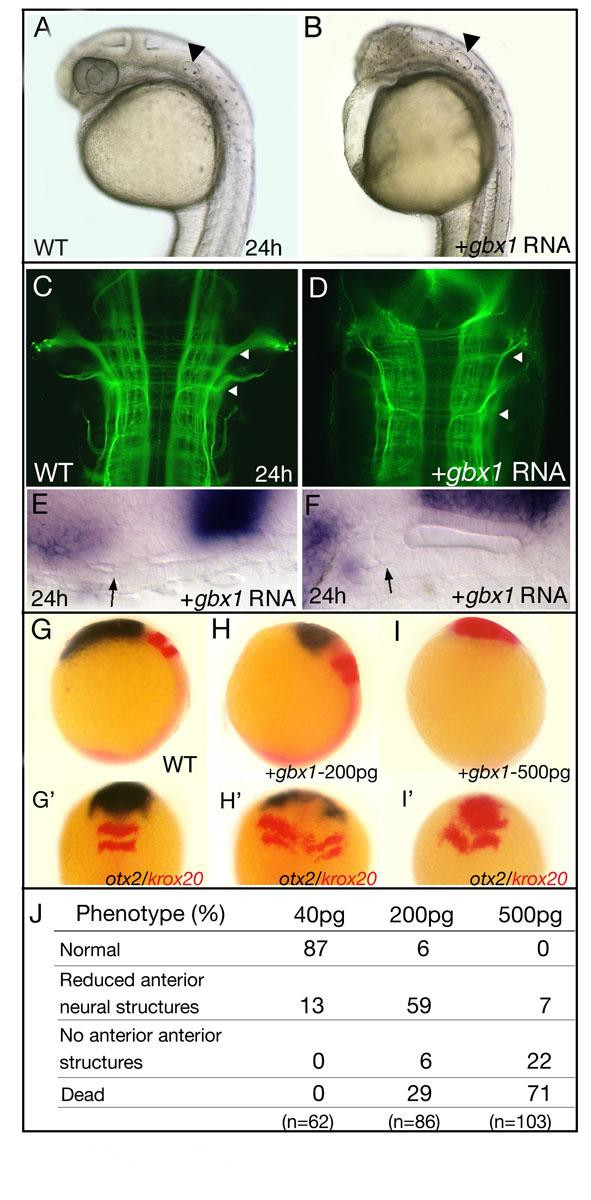
Overexpression of gbx1 induces posterior cell fate. (A) Wild-type (WT) embryo at 24 h and (B) after injection of 200 pg gbx1 mRNA; anterior brain structures are severely reduced. The ear is indicated by an arrowhead. (C, D) Staining of the forming axon tracts with an anti-acetylated tubulin antibody at 24 h; dorsal views, with anterior to the top. In the gbx1-injected embryo the hindbrain is severely enlarged compared to WT (arrowheads). (E, F) Duplications of ear structures are frequently observed (arrows). (G-I') Series of embryosinjected with different doses of gbx1 mRNA (200 and 500 pg), analyzed at the tailbud stage after in situ hybridization with otx2 (blue) and krox20 (red). The otx2 domain progressively disappears and the krox20 domains shift to more anterior regions. (J) Dose-dependent gbx1 overexpression phenotypes. Higher concentrations (>500 pg) did not increase the observed phenotype. (A-D, K-M) Lateral views; (E-H, K'-M') dorsal views.
To study the effect of gbx1 overexpression during neural plate regionalization, we analyzed gbx1-injected embryos at the tailbud stage using otx2 as a forebrain/midbrain marker, and krox20 as a hindbrain marker specific for rhombomeres (rh) 3 and 5. Increasing amounts (40–500 pg) of injected gbx1 mRNA gradually removed all anterior neural fates (Figure 1G–J), with the highest concentrations resulting in rh3 occupying the rostral end of the embryo (Figure 1G–I'). In all cases, otx2 expression was strongly reduced or absent, whereas krox20 expression was expanded (Figure 1G–I'). This expansion was associated with rh3 rather than rh5 (Figures 1I' and 2Q, R), suggesting that rh3 is more sensitive to gbx1. The absence or reduction of otx2 was already observable at 60% epiboly (Figure 3E, E').
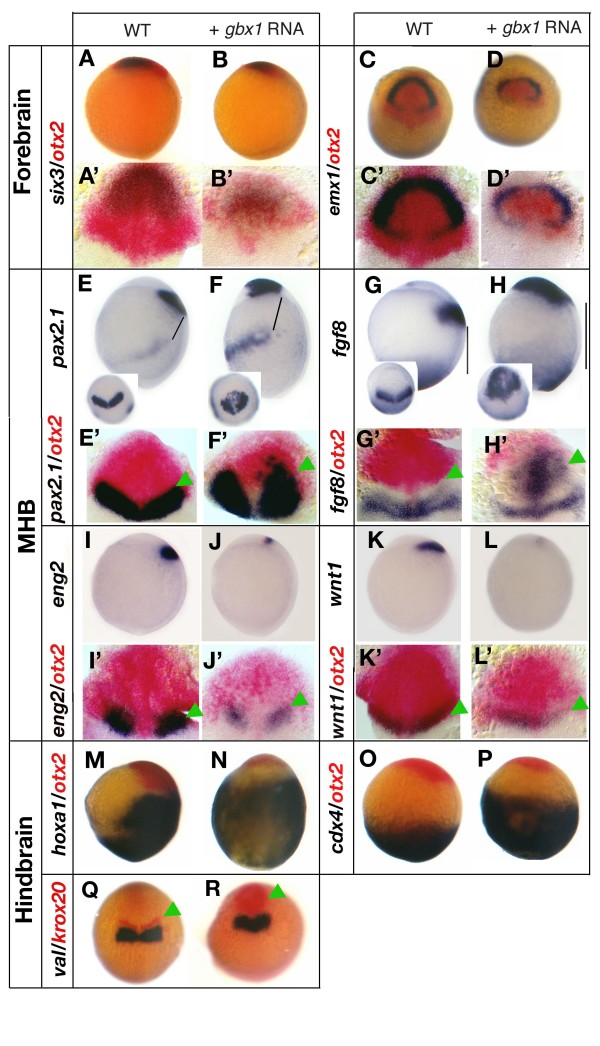
Figure 2.
gbx1 overexpression affects midbrain, midbrain-hindbrain boundary (MHB) and hindbrain. (A, A') Control embryo stained for six3 (blue) and otx2 (red);. (B, B') gbx1-injected embryo. The near absence of otx2-positive cells posteriorly to the six3-expressing domain indicates a loss of the midbrain territory in the gbx1-injected embryos. (C, C') Control embryo stained for emx1 (blue) and otx2 (red). (D, D') gbx1-injected embryo. The emx1 domain corresponding to forebrain remains robust, whereas the otx2 domain corresponding to midbrain is lost. (E) Wild-type (WT) embryo after in situ hybridization (ISH) with pax2.1 and (E') with pax2.1 and otx2 (red). (F, F') gbx1-injected embryos; pax2.1 is expressed ectopically in the anterior of the embryo. The MHB is shifted anteriorly as indicated by green arrowheads. Also, the distance between the posterior pax2.1 domain and the anterior domain is increased (black bars in (E, F)). (G) WT embryo after ISH with fgf8 and (G') with fgf8 and otx2 (red). (H, H') gbx1-injected embryos; fgf8 is expressed ectopically in the anterior of the embryo. The MHB is shifted anteriorly as the distance between this expression domain and the margin in increased (black bars in (G, H)). (I) WT embryo after ISH with eng2 and (I') with eng2 and otx2 (red). (J) gbx1-injected embryo; eng2 is not expressed ectopically. (J') Combined ISH with otx2 (red) shows that the remaining domain is located caudally to the reduced otx2 domain. (K) WT embryo after ISH with wnt1 and (K') with wnt1 and otx2 (red). (L) gbx1-injected embryo;wnt1 is not expressed ectopically. (L') In all gbx1-injected embryos the MHB is shifted anteriorly as indicated by green arrowheads. (M) WT embryo after ISH with hoxa1 and otx2 (red). (N) gbx1-injected embryo; the hoxa1 expression doamin is enlarged. (O) WT embryo after ISH with cdx4 and otx2 (red). (P) gbx1-injected embryo; the cdx4 expression domain is enlarged. (Q) WT embryo after ISH with val and krox20 (red). (R) gbx1-injected embryo; val expression is not affected: the krox20 expression domain corresponding to rh3 (green arrowhead) is enlarged, whereas rh5 is less affected. (A, B, E-P) Lateral views, anterior to the left; (C, D-Q, R) dorsal views, anterior to the top; (A'-L') flat-mounted embryos, anterior to the top.
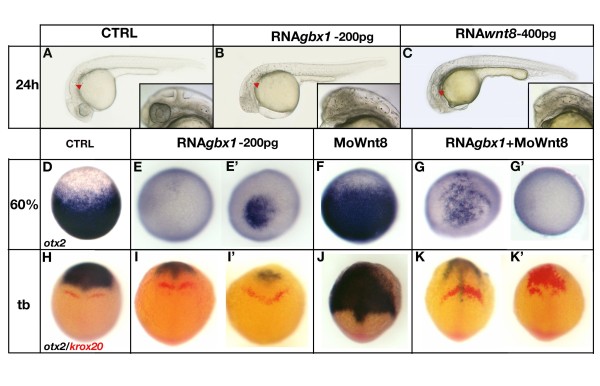
Figure 3.
gbx1 acts as a mediator of the Wnt8 hindbrain posteriorizing signal. (A) Control embryo at 24 h, (B) gbx1-injected embryo (200 pg), (C) wnt8-injected embryo (400 pg). gbx1 overexpression mimics wnt8 gain-of-function; the embryos are truncated anteriorly. The red arrowhead indicates the position of the ear. (D-G') Animal pole views at 60% epiboly after ISH with otx2. (D) Control embryo, (E, E') gbx1-injected embryos (200 pg), (F) embryo injected with wnt8 morpholinos, (G, G') embryos co-injected with gbx1 mRNA and wnt8 morpholinos. Expansion of the otx2 domain in the wnt8 morphant is rescued by co-injection with gbx1 mRNA. (H-K') Dorsal views at the tailbud (tb) stage after ISH with otx2 (blue) and krox20 (red). (H) Control embryo, (I, I') gbx1-injected embryos, (J) embryo injected with wnt8 morpholinos, (K, K') embryos co-injected with gbx1 mRNA and wnt8 morpholinos. Loss of krox20 and posterior expansion of the otx2 domain in the wnt8 morphant is rescued by gbx1 mRNA.
In sum, these data show that ectopic gbx1 represses otx2 expression, reduces tissue with forebrain/midbrain identity and increases the amount of tissue with posterior identity (hindbrain, ear). We conclude that gbx1 has posteriorizing activity at early stages of development, acting during hindbrain development and, as suggested by its repression of otx2, during demarcation of the MHB primordium.
gbx1 induces posterior cell fate by transforming anterior neural tissue
The observed loss of anterior neural tissue and expansion of posterior neural tissue in gbx1-injected embryos could result from either transformation of anterior tissue towards a posterior fate, or a loss of anterior tissue due to cell death. To distinguish between these possibilities, we used a labeling technique to follow small cell clones [33] in the presumptive midbrain neural plate of gbx1-misexpressing embryos, and to map their location at 20 h of development. Embryos were injected at the one-cell stage with a caged-fluorescent dye that was later uncaged in a small group of cells at the appropriate stage by a nitrogen laser emitting a wavelength of 365 nm (Figure 4A). In embryos co-injected with 200 pg gbx1-mRNA, we activated fluorescein at 60% epiboly in a group of cells within the prospective otx2 domain as determined by the established fate map [34]) (Figure 4B, C). The fate of these fluorescent cells was then followed throughout development. At 20 h, the labeled cells sat in the rostral part of the embryo, close to the otic vesicle (Figure 4D, E), suggesting that although these cells were fated to become anterior, gbx1 overexpression transformed them into a posterior identity. In addition, TUNEL analysis of gbx1-injected embryos at the tailbud stage showed no difference in the distribution of apoptotic cells compared to control embryos, suggesting that there is no increase in cell death (data not shown). We therefore conclude that gbx1 is capable of repressing midbrain/forebrain identity within the neuroectoderm, most likely expanding posterior neural tissue via the transformation of anterior neural fates. This supports a role for gbx1 in specifying hindbrain cell fate.
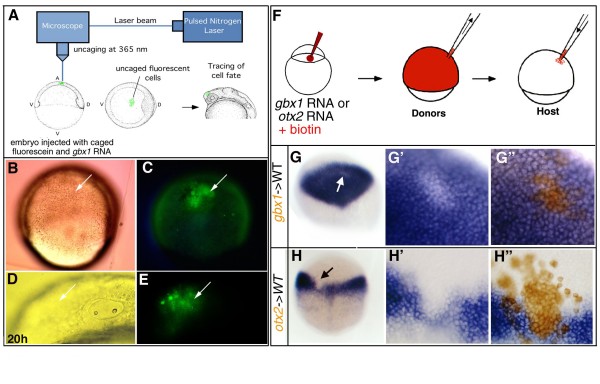
Figure 4.
gbx1 induces posterior neural fate via transformation of anterior neural fate and gbx1 and otx2 repress each other cell-autonomously. (A) Schematic drawing of the fluorescein uncaging procedure (for details, see [33]). (B, C) gbx1-injected embryo after uncaging cells in the prospective otx2 domain. (D, E) After 20 h of development the labeled cells come to lie in the anterior of the embryo (arrow). (B, D) Nomarski optics; (C, E) detection of the fluorescent cells (arrow). (F) Experimental procedure for cell transplantations. Donor embryos are generated by co-injecting biotinylated dextran as lineage tracer and gbx1 (300 pg) or otx2 mRNA (300 pg). Cells are taken out of the donor at 40% epiboly and transplanted into a wild-type (WT) host embryo. (G) Chimeric embryo containing cells derived from embryos injected with biotinylated dextran and gbx1mRNA, and stained for otx2 (blue). Unlabeled patch of cells marked by a white arrow. (G') Close-up of the patch indicated in (G) before biotin staining and (G") after biotin staining (brown). gbx1 overexpressing cells within the otx2 domain do not express otx2. (H) Chimeric embryos containing cells derived from embryos injected with biotinylated dextran and otx2 mRNA, and stained for gbx1 (blue). Unlabeled patch of cells marked by a black arrow. (H') Close-up of the patch indicated in (H) before biotin staining and (H") after biotin staining (brown). otx2 overexpressing cells within the gbx1 domain do not express gbx1.
gbx1 and otx2 repress each other cell-autonomously
The observed repressive action of gbx1 led us to ask whether gbx1 acts cell-autonomously to repress otx2. To address this, gbx1-overexpressing cells (300 pg) were transplanted into the prospective otx2 domain of wild-type embryos at 50% epiboly, and assayed for otx2 expression at 80% epiboly (Figure 4F–G"). Transplanted gbx1-positive cells that came to lie within the otx2 expression domain did not express otx2, whereas all adjacent gbx1-negative cells were otx2 positive (Figure 4G–G"; n = 15). This indicates that the absence of otx2 expression in gbx1-expressing cells is a cell-autonomous effect.
As mutual repressive interactions may exist between Otx and Gbx genes (see Introduction; reviewed in [10,11,26]), we next asked if otx2 can repress gbx1 expression in a cell-autonomous manner. For this purpose, we ubiquitously overexpressed otx2 by injecting mRNA in one-cell stage embryos. Although these embryos showed aberrant epiboly and gastrulation movements (data not shown) due to the action of otx gene products in embryonic cell aggregation [35,36], we could observe that gbx1 expression was strongly diminished (data not shown). To evaluate the interaction between otx2 and gbx1 in the context of a normal gastrulating embryo, we transplanted otx2-overexpressing cells (300 pg) at 50% epiboly into the prospective gbx1 domain of wild-type embryos and assayed for gbx1 expression at the tailbud stage (Figure 4F, H–H"). The transplanted otx2-positive cells that came to lie within the gbx1 expression domain did not express gbx1, but all adjacent otx2-negative cells were gbx1 positive (Figure 4H–H"; n = 10). This result suggests that the repression of gbx1 in otx2-expressing cells is a cell-autonomous effect.
Altogether, these results show that zebrafish gbx1 and otx2 can mutually repress each other in an autonomous manner. This supports the idea that, as described in the mouse for Gbx2 and Otx2, such mutual exclusion may be a mechanism to subdivide the neural plate into juxtaposed otx and gbx expression domains that will be required to position the MHB.
gbx1 overexpression acts in a concentration-dependant manner to transform midbrain and forebrain territories
In the above experiments, we found that increasing amounts of injected gbx1 mRNA gradually removed all anterior neural fates: intermediate concentrations reduced the otx2 expression domain (Figure 1H, H') and higher concentrations completely abolished it (Figure 1I–I'). This suggested that not all regions of the otx2 domain are equally sensitive to gbx1, perhaps because of differences between fore- and midbrain response.
To investigate this possibility, we injected intermediate doses of gbx1 mRNA in order to retain residual otx2 expression (Figure 1H–H'), and followed the identity of this otx2 domain by analyzing fore- and fore/midbrain specific marker expression using dual labeling. Analysis of the forebrain marker six3 showed that the majority of the residual otx2 domain is six3-positive, leaving only a few cells that express otx2 alone (Figure 2A–B'). This suggests that the otx2-positive territory in gbx1-injected embryos largely possesses a forebrain identity and that the midbrain identity is almost completely lost. We also found that another forebrain marker, emx1, was partly reduced (Figure 2C–D'). Noticeably, two bilateral transverse emx1 stripes were still visible in the posterior forebrain (diencephalic primordium) [37], suggesting that the posterior forebrain is present.
Together, these findings indicate that ectopic gbx1 at relatively low doses leads to local transformation that affects the midbrain, the territory adjacent to its normal expression domain; only high doses of gbx1 transform forebrain into more posterior neural fates. These data suggest differences in fore- and midbrain response to gbx1 and further support a role for gbx1 in positioning the otx2 posterior border at which the MHB primordium forms.
gbx1 overexpression shifts the midbrain-hindbrain boundary anteriorly
Previous analysis of gbx1 expression in wild type and in noi/pax2.1, spg/pou2 and ace/fgf8 mutants suggested that gbx1 might act upstream of these genes as an important regulator of MHB formation, particularly during the maintenance phase [8,38]. Therefore, the effect of gbx1 overexpression on these MHB markers was examined. Analysis of pax2.1, which is expressed at the tailbud stage in the posterior midbrain and the anterior hindbrain [8,13], and fgf8, expressed in the anterior hindbrain with its anterior border abutting the otx2 expression domain [39], showed that these genes were activated ectopically in the anterior neural plate when gbx1 was overexpressed (Figure 2E–H'). This phenotype was observed with high frequency when 200 pg of gbx1 RNA was injected. In the gbx1-injected embryos, the pax2.1 mid-hindbrain expression domain shifted anteriorly as the distance between this domain and the lateral plate mesoderm expressing domain increased (compare the black bars in Figure 2E, F). Furthermore, the anterior limit of the fgf8 expression domain, which corresponds to the MHB, also shifted anteriorly as the distance between this domain and Fgf8-expressing cells at the margin increased (Figure 2G, H). pax2.1 and fgf8 expression was also analyzed after dual labeling with an otx2 probe (red), which showed that pax2.1 and fgf8 domains retain a normal spatial relationship with respect to the otx2 posterior border (Figure 2E'–H', green arrowheads). The overall anterior displacement of the otx2/pax2.1 and otx2/fgf8 domains further shows that ectopic gbx1 expression is able to relocate the MHB.
wnt1 and eng2 also belong to the set of genes expressed at the gbx1/otx2 interface and required for MHB organizer maintenance. wnt1 is expressed at the tailbud stage in the posterior midbrain where it overlays the posterior border of otx2 [40], and eng2 is expressed in the posterior midbrain and anterior hindbrain [41,42]. In contrast to fgf8 and pax2.1, wnt1 and eng2 were not ectopically induced in gbx1-injected embyos (Figure 2I–L'). Both genes' expression domains were reduced in the gbx1-overexpressing embryos. Co-labeling with otx2 showed that their residual expression was located anteriorly within the neural plate, retaining a normal spatial relationship with the otx2 posterior border (Figure 2I'–L", green arrowheads) and marking the abnormally anteriorly positioned MHB.
Our results demonstrate that changing the A-P position of the gbx1/otx2 interface, by ectopically expressing gbx1, consistently changes the position of early MHB marker onset (fgf8, pax2, 1, wnt1 and eng2) and, thus, the location of the developing MHB organizer.
gbx1 overexpression mainly expands the anterior hindbrain territory
Previous analysis of gbx1 expression showed that it is also expressed in the hindbrain and spinal cord primordium from 60% epiboly onwards [8]. Morphological (not shown) and in situ hybridization (ISH) analysis of krox20 in gbx1-injected embryos (Figure 1) revealed that the posterior part of the embryo was enlarged, prompting us to further study hindbrain development. For this we looked at hoxa1, which is expressed in the hindbrain with an anterior limit corresponding to the border rh3/rh4 [43], valentino (val), which is expressed in rh5 and rh6 [44], and a caudal-related gene, cdx4 [45], which is expressed in the posterior third of the embryo[45]. In gbx1-injected embryos, the hoxa1 expression domain is strongly expanded (Figure 2M, N), whereas the val expression domain remains stable (Figure 2Q, R). Interestingly, in the same embryo krox20 expression in rh3 (red) is expanded (Figure 2Q, R). This shows that all rostral boundaries up to rh3 are moved rostrally and keep their relative position to each other. The posterior hindbrain (val domain) seems less sensitive to gbx1 overexpression. cdx4 expression domains is strongly expanded (Figure 2O, P), supporting an enlargement of the prospective spinal cord territory.
gbx1 mediates the Wnt8 hindbrain posteriorizing signal
Previous studies have shown that Wnt8, a posteriorizing factor, acts upstream of gbx1 [32], and that Wnt8 loss-of-function strongly impairs hindbrain and spinal cord development [46-48]. Interestingly, like gbx1, ectopic wnt8 activation is known to expand posterior neural fates, resulting in anterior head truncation (Figure 3C) [46,48,49]. We found that gain-of-function of either gene truncated the entire forebrain/midbrain (Figure 3A–C), while expanding the hindbrain and shifting the otic vesicle anteriorly (Figure 3A–C, red arrowheads). If Wnt8 induction of gbx1 expression is instrumental in mediating Wnt signaling effects on neuroectoderm patterning, the loss of Wnt8 may be rescued by restoring gbx1 function. To test this model, we investigated if gbx1 can rescue the loss of posterior tissue in wnt8 morphants by co-injecting wild-type embryos with gbx1 mRNA (200 pg) and wnt8 antisense morpholinos [32,48]. As previously described, wnt8-morphants display a ventro-laterally expanded otx2 expression domain (Figure 3F, dorsal views) [32,48], and gbx1-injected embryos lack almost all otx2 at 60% epiboly (Figure 3E, E'). Co-injection of wnt8 morpholinos and gbx1 mRNA resulted in a loss of otx2 expression (50%, n = 20) or a faint patchy expression (50%, n = 20) (Figure 3G, G'), suggesting that gbx1 reduced or abolished the otx2 expansion observed in the wnt8 morphants. Similar effects on otx2 levels were observed at the tailbud stage (Figure 3H–K', blue labeling). At this stage, loss of krox20 expression was also seen in wnt8 morphants (65%, n = 50; Figure 3J). Co-injection with gbx1 mRNA rescued this krox20 domain, which, however, was mislocated at the A-P level (90%, n = 40; Figure 3K, K').
Together, these findings demonstrate that gbx1 can partially restore hindbrain patterning in the absence of Wnt8, suggesting that gbx1 compensates for loss of Wnt8. Considering that Wnt8 is required for the onset of gbx1 expression, as well as for the correct A-P positioning of its expression domain [32], we propose that gbx1 acts at the transcriptional level to mediate Wnt8 posteriorizing effects on hindbrain patterning. In view of the large hindbrain gbx1 expression domain [8], this gbx1 function in hindbrain patterning could be independent of its role in MHB positioning.
gbx1 loss-of-function reduces the antero-posterior extent of the hindbrain/spinal cord territory
We next investigated the effect of gbx1 loss-of-function on zebrafish development by inhibiting mRNA translation with antisense morpholinos [50]. Two non-overlapping morpholinos, Mo1 and Mo2, were designed in the 5' untranslated region of gbx1. Injecting either of these morpholinos produced similar morphological phenotypes (Additional file 1A–F). When Mo1 was injected at a low dose (1–5 ng), the embryos developed properly, displaying only weak MHB abnormalities at 24 h. At an elevated dose (5–10 ng), embryonic development slowed during gastrulation such that, from the tailbud stage, the injected embryos failed to elongate properly (Additional file 1D, E). At 24 h, the anterior brain region of the injected embryos was highly affected and the A-P extent of the hindbrain was reduced (Additional file 1D, E). Mo2 generated the same phenotypes already at lower doses (Additional file 1D, F)
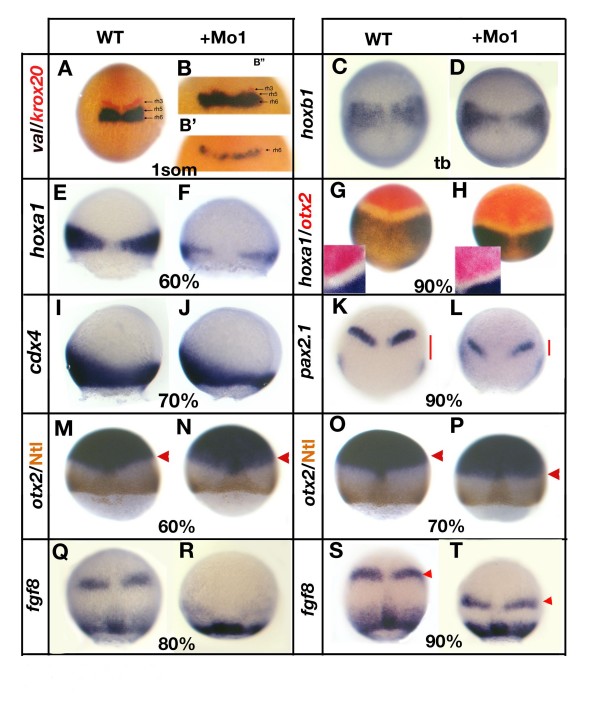
Considering that gbx1 is initially expressed in the anterior hindbrain and spinal cord primordium, we first investigated whether these territories are affected in embryos lacking gbx1 protein. We injected morpholinos at concentrations that maximally blocked translation (10 ng of Mo1, 5 ng of Mo2; Figure 5G, H). Because the gbx1 morphants developed large amounts of necrosis at 20–24 h (Additional file 1D–F), we focused our analysis on stages from gastrulation to five somites. We used krox20 to mark rh3 and rh5, and otx2 to mark the forebrain/midbrain. Embryos were co-labeled with myoD, a basic helix-loop-helix (bHLH) transcription factor expressed in adaxial mesoderm and the forming somites, allowing us to properly stage the embryos. In Mo1-injected embryos stained for otx2/krox20/myoD at the four-somite stage, we found that the distance between the posterior border of otx2 and the rostral limit of myoD was smaller along the A-P axis (Figure 5A–B', red bars). Also, the overall length of the myoD expression domain was shorter, suggesting a reduction in spinal cord length. Injection of Mo1 or Mo2 led to a complete loss of krox20 in 50% of the embryos (Figure 5B, C). In the other 50%, only one stripe was present and its width was reduced in comparison with the wild-type krox20 stripes (Figure 5A–C'). The use of myoD to stage the embryos, allowing the somites to be counted, confirms that the presence of only one krox20 stripe is not the result of delayed development, and further analysis (see below) confirmed the rh3 stripe is missing (Additional file 2). Overall, these data indicate that loss of gbx1 affects the anterior hindbrain and spinal cord.
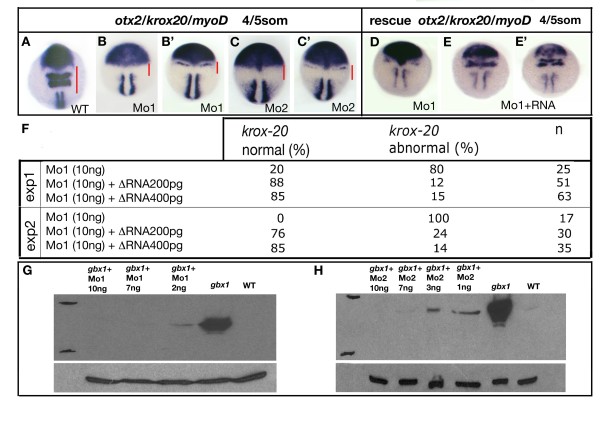
Figure 5.
gbx1 morpholinos affect hindbrain patterning. (A-C') Embryos at the four somite stage stained for otx2/krox20/myod. (A) Control embryo, (B, B') embryos injected with 10 ng Mo1, (C, C') embryos injected with 7 ng Mo2. Injections of two different morpholinos show the same phenotype morphologically and after in situ hybridization analysis. (D-F) Rescue experiments using the krox20 expression domain as a read-out. (D) gbx1-morphant, (E, E') rescued morphant embryos co-injected with gbx1 mRNA that does not contain the MO binding sequence in its 5' region. (F) Two combinations were tested: 10 ng of Mo1 with 200 pg or 400 pg of gbx1 mRNA. (G, H) Western blot detection showing down regulation of gbx1-myc mRNA translation in embryos co-injected with gbx1-myc mRNA containing morpholino binding sequences and Mo1 (G) or Mo2 (H). WT, wild type.
Our next step was to validate gbx1 morpholino specificity by performing two sets of experiments. First, co-injection of individual morpholinos and gbx1-myc tagged mRNA containing sequences for morpholino binding was followed by protein extraction and western blot detection of the myc epitope, which confirmed that both Mo1 and Mo2 efficiently block translation of gbx1 mRNA (Figure 5G, H). Because more Mo2 than Mo1 had to be injected to obtain the same degree of inhibition, and Mo2 induced high levels of general necrosis, Mo1 was utilized for all further experiments. In our second test of morpholino specificity, we attempted to rescue morphants with a synthetic gbx1 mRNA that cannot be bound by the morpholino (Dgbx1), and analyzed krox20 domains as a read-out (Figure 5D–F). Two combinations were used: 10 ng of Mo1 plus either 200 pg or 400 pg of Dgbx1 mRNA. As shown in Figure 5F, co-injected embryos expressed krox20 in two distinct stripes, whereas almost no expression was seen in embryos injected with Mo1 alone (Figure 5F). This rescue by Dgbx1 RNA suggests that the loss of krox20 expression is specific to the loss of gbx1 protein, rather to secondary effects of the morpholino. Not surprisingly, the observed necrosis at 24 h could not be rescued by co-injection with Dgbx1 mRNA, indicating that this phenotype could be attributed to secondary morpholino effects.
Our combined results therefore support the specificity of the gbx1 morpholino phenotype: first, identical phenotypes were observed with two different morpholinos (Figure 5A–C'); second, western blot analysis demonstrated that both Mo1 and Mo2 block translation of gbx1-myc mRNA (Figure 5G, H); third, we could rescue loss of krox20 expression using gbx1 mRNA (Figure 5D–F).
The loss of krox20 expression in the hindbrain of embryos injected with gbx1 morpholino suggests that some rhombomeres are not specified properly. To investigate this we looked at valentino (val), a marker of rh5 and rh6. In 50% of gbx1 morphants, we observed the two stripes corresponding to val expression in rh5 and rh6 (Figure 6A, B, B'). Co-staining with krox20 shows a faint red band anteriorly to the two blue stripes corresponding to the remaining expression of krox20 in rh3 (Figure 6A, B, B'). In the other 50% of gbx1 morphants, only one stripe of val was observed and co-staining with krox20 indicated that the remaining stripe corresponds to rh6 (Figure 6B"). Thus, we suggest that krox20 expression is lost in rh3 and that only in the most affected morphants is its expression is lost in rh5. This corroborates our gain-of-function experiments where we observed that rh3 is more sensitive to gbx1. We then analyzed expression of several hindbrain markers in gbx1 morphants at 60–90% epiboly. We found reduced hoxb1 expression in rh4 and spinal cord, suggestive of a shortened hindbrain/spinal cord domain (Figure 6C, D). Expression of hoxa1 at 60% epiboly (Figure 6E, F) and the tailbud stage (Figure 6G, H), and cdx4 at 70% epiboly (Figure 6I, J), also indicated hindbrain reduction. Furthermore, the size of the gap between otx2 and hoxa1 expression domains was reduced (Figure 6G, H), suggesting that gbx1 is required locally for hindbrain specification.
Figure 6.
Loss of gbx1 affects hindbrain and midbrain-hindbrain boundary (MHB) markers. (A-H, M-K-T) are dorsal views, (I, J) are lateral views and the developmental stages are indicated (tb, tailbud stage). (A) Control embryo (wild type (WT)) stained with val (blue) and krox20 (red), (B, B') gbx1-morphants. A residual krox20 expression is seen in (B') and no expression of krox20 in (B"). For (B', B") a higher magnification of the region of interest is shown. (C) Control embryo stained with hoxb1; (D) gbx1-morphant. (E) Control embryo stained with hoxa1; (F) gbx1-morphant. (G) Control embryo stained with hoxa1 and otx2; (H) gbx1-morphant. A higher magnification is shown, illustrating the reduction of size of the gap between the otx2 and hoxa1 domains. (I) Control embryo stained with cdx4; (J) gbx1 morphant. (K) Control embryo stained with pax2.1; (L) gbx1-morphant. The red bars show the distance between the MHB and the lateral plate mesoderm expression domains. This distance is markedly reduced in the gbx1 morphants. (M, O) Control embryos stained with otx2 (blue) and Ntl antibody (brown); (N, P) gbx1 morphants. The otx2 domain is unaffected at 60% epiboly but slightly shifted posteriorly at 70% (red arrowheads). (Q, S) Control embryos stained with fgf8; (R, T) gbx1-morphants. MHB expression is delayed (R) and shifted posteriorly (S, T, red arrowheads) in the morphants.
Altogether, the analysis of various markers in gbx1-morphants reveals a shortening of the hindbrain and spinal cord that suggests a reduction in posterior neural fates. These observations complement our gain-of-function results, thereby supporting a role for gbx1 in mediating posteriorization signals that pattern the hindbrain.
gbx1 loss-of-function shifts the midbrain-hindbrain boundary posteriorly
Having found that gbx1 overexpression relocates the MHB primordium more anteriorly, we next sought to assess early neural plate development in gbx1 morphants. Examination of the otx2 domain together with Ntl antibody staining to visualize the margin of the embryo in the gbx1 morphants showed no abnormalities at 60% epiboly (Figure 6M, N). However, starting at 70% epiboly, a slight caudal shift of the posterior otx2 border was observed (Figure 6O, P). We have previously shown that the gbx1 and otx2 expression domains overlap by three to four cell rows at 60%; however, at 70% the two domains abut each other without overlap [8]. This suggests that gbx1 is required to correctly establish the otx2 posterior border starting at 70% epiboly. Additionally, it indicates that there is a phase when the otx2 and gbx1 domains are established independently of each other before they interact.
To determine the role of gbx1 in MHB development, we examined the effect of gbx1 loss-of-function on MHB gene expression. fgf8 (Figure 6Q–T), pax2.1 (Figure 6K, L), wnt1 and eng2 (not shown) were all expressed in gbx1-morphants, although fgf8 was slightly delayed (Figure 6Q, R). This suggests that Gbx1 protein is not required for initial MHB marker expression and, by extension, for the establishment of MHB organizing activity. The presence of MHB gene expression also indicates that the maintenance regulatory loop functions correctly in the absence of Gbx1. Nonetheless, the A-P position of fgf8 (Figure 6S, T) and pax2.1 (Figure 6K, L) MHB domains was posteriorly shifted in gbx1 morphants. At 80% epiboly, morpholino injections led to a reduction in the A-P distance between fgf8 expression domains in the MHB primordium and the margin (Figure 6S, T, red arrowheads). Similarly, in gbx1 morphants at 80% epiboly, the distance between pax2.1 domains in the MHB primordium and the lateral plate mesoderm was decreased (Figure 6K, L, red bars) and the expression was reduced in both domains. Thus, our results indicate that in the absence of Gbx1 protein, the otx2 expression domain shifts slightly to the posterior and leads to a similar displacement of the MHB as reflected by shifts in fgf8 and pax2.1.
Juxtaposition of gbx1 and otx2 can induce MHB markers
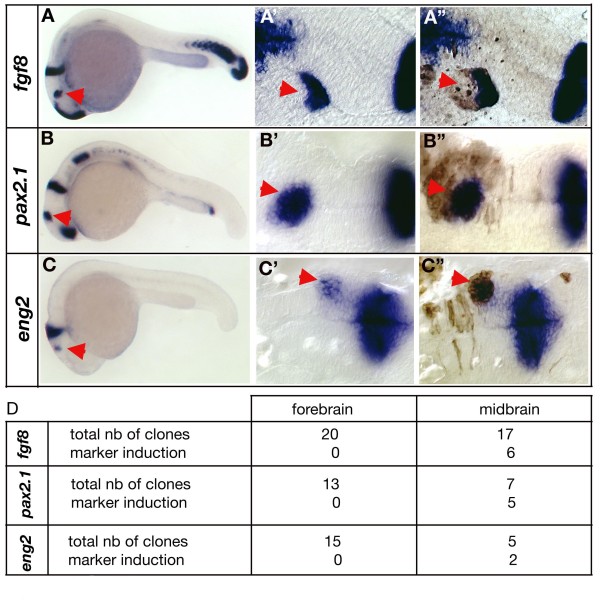
Our data reveal that an interaction between otx2 and gbx1 defines the position of the MHB organizer in the early neural plate. Given this, ectopic juxtaposition of an otx2 and gbx1 domain should trigger the expression of MHB markers. To test this hypothesis, we transplanted cells from gbx1-injected embryos (500 pg) into the otx2 domain of wild-type embryos to generate an ectopic otx2/gbx1 interface, and then looked at markers for MHB induction. We found that three MHB markers, fgf8, pax2.1 and eng2, were induced at this ectopic interface at 24 h of development (Figure 7). Induction of these markers was preferentially observed in the more posterior otx2 domain (Figure 7A–C", arrowheads) and correlated with the size of the clone, as small clones or single cells did not induce ectopic MHB marker expression. We also observed that clones localized in the forebrain territory never induced MHB markers (Figure 7D). It was previously found that neither isthmus grafts nor Fgf8 beads placed in prosomere 2 (diencephalon) induce host tissue to form cerebellum [51,52]. Our observations agree with this finding in suggesting that additional factors expressed at the MHB level are required together with otx2 and gbx1 to initiate MHB marker expression.
Figure 7.
The otx2/gbx1 interface plays an essential role in midbrain-hindbrain boundary development. (A) Lateral view of a chimeric embryo stained for fgf8. The red arrowhead indicates ectopic fgf8 expression. (B) Lateral view of a chimeric embryo stained for pax2.1. The red arrowhead indicates ectopic pax2.1 expression. (C) Lateral view of a chimeric embryo stained for eng2. The red arrowhead indicates ectopic eng2 expression. (A', B', C') Close-up of the ectopic patch before biotin staining and (A", B", C") after biotin staining (brown). For all the three genes, ectopic expression is induced in gbx1-overexpressing cells within the otx2 domain (red arrowheads). (D) Summary table of the transplanted cells' positions and marker induction. Clones localized in the forebrain never induced ectopic fgf8, pax2.1 or eng2 expression.
As a whole, these results show that a MHB molecular cascade, including fgf8, pax2.1 and eng2, is selectively triggered at the point of ectopic juxtaposition of otx2 and gbx1 domains in a competent neuroepithelium in zebrafish.
Discussion
Previous studies have shown that in amphibian and amniotes (chick, mouse, Xenopus), mutual repression between Otx2 and Gbx2 at the future MHB results in the positioning and sharpening of the Otx2/Gbx2 border (reviewed in [10,26]). Similar to Gbx2 expression in mouse [7,29], one of the zebrafish gbx genes, gbx1, is expressed adjacent to the otx2 domain at the end of gastrulation [8]. This led us to test if gbx1 plays a role in early neural patterning. Here we show that gbx1 does indeed play an important role in conjunction with otx2 to establish the MHB. Additionally, we describe a novel role for gbx1 as a mediator of the Wnt8 posteriorization signal required for hindbrain development. Thus, our work implicates gbx1 in two different aspects of early neural plate patterning.
gbx1 represses otx2 expression and positions the MHB organizer
Our functional analysis supports the hypothesis that mutual repression between gbx1 and otx2 serves as a mechanism for MHB positioning. Ectopic gbx1 represses otx2 expression and repositions the MHB at the new gbx1/otx2 border. This is evidenced by an anterior shift in MHB marker gene expression, including the ectopic induction of pax2.1 and fgf8 in the anterior brain. Conversely, gbx1 loss-of-function allows the posterior expansion of the otx2 expression domain and repositions the MHB posteriorly. Considering our finding that pax2.1, fgf8, eng2 and wnt1 are expressed in gbx1 morphants, we suggest that, as in mouse [15-19], the activation and maintenance of MHB gene expression is independent of both otx and gbx1 function. Interestingly, although they are mislocated in gbx1 morphants, the fgf8 and pax2.1 domains are not expanded in gbx1 morphants and retain their shape. This could be due to gbx2 functioning later in development [8] to repress otx2 at its most posterior position and thereby keep its posterior border sharp.
Our gain-of-function experiments support the idea that gbx1 is involved in activation of pax2.1 and fgf8; however, it is clear from other work that gbx1 cannot directly activate pax2.1 expression. We previously observed that onset of pax2.1 expression occurs outside the endogenous gbx1 domain [8], and most likely requires an additional, diffusible signal. Co-injection of gbx1 and the Wnt inhibitor dickkopf-1 (dkk1), followed by staining for pax2.1 expression at 80% epiboly, showed that pax2.1 is not activated (MR and MB, unpublished results), suggesting that Wnt signals may be involved in the activation of pax2.1. Such signals could be wnt8b, wnt1 and/or wnt10b [46,53], all of which are expressed just before or during the onset of pax2.1 expression at the MHB. One explanation for ectopic pax2.1 activation in gbx1 overexpressing embryos is the fact that all A-P information, including pax2.1 activation signals, is shifted to more anterior regions.
Contrary to a general effect on MHB genes, wnt1 and eng2 are not ectopically expressed in gbx1-overexpressing embryos, but are instead downregulated. The failure of gbx1 to induce ectopic eng2 expression was indeed surprising considering that an earlier analysis of noi/pax2.1 mutants has shown that pax2.1 is necessary for eng2 activation [13]. It is possible that additional (co)factors, not induced in the gbx1-overexpressing embryos, might be involved. Indeed, it has been shown that Xenopus engrailed-2 is a direct target of Wnts [54], hinting at a link between wnt1 and eng2 diminished expression. Downregulation of wnt1 in gbx1-injected embryos is most likely the consequence of otx2 repression. In mouse, it has been shown that Otx2 is required cell autonomously for Wnt1 activation [15] and our data in zebrafish show that this pathway may be maintained, although it cannot be excluded that gbx1 directly represses wnt1 expression.
otx2 and gbx1 interact to refine their respective expression domains
Our gbx1 loss-of-function data show that the otx2 domain remains robust at 60% epiboly but shifts slightly posterior at 70% epiboly. This is consistent with otx2 and gbx1 domains being independently established and interacting later to refine their respective expression domains. These data correlate with the observation in mouse that the presence of Gbx protein is required for refinement and maintenance of the Otx2 expression domain, but not its onset [17,18,55]. Most likely this refinement function in zebrafish and mouse does not act to position the expression domains globally within the neural plate. This level of positioning is controlled by the secreted Wnt8 molecule [32].
The observed sharpening of otx2 and gbx1 expression domains between 60% and 80% epiboly marks a period during which mutually repressive interactions are likely to take place [8]. Interestingly, a key step in the induction of posterior neural fate in gbx1-overexpressing embryos seems to be repression of otx2 expression. Our cell transplantation studies have shown that this otx2 repression occurs cell-autonomously within the injected cells. Conversely, otx2 cell-autonomously represses gbx1. These results are consistent with the mutual repression of Otx2 and Gbx2 observed during mis-expression experiments in mice, Xenopus and chicken [17,20,21,25] and suggests that whichever Gbx gene is expressed at a higher level or at the proper timing determines cell fate.
The otx2/gbx1 interface in zebrafish is equivalent to the Otx2/Gbx2 interface in mouse
Several similarities are observed between zebrafish gbx1 and mouse Gbx2 (or chicken and Xenopus). First, in mouse and chicken, Gbx2 is expressed during gastrulation and its expression domain does not immediately abut the Otx2 expression domain [55,56]. Only at the end of gastrulation are two distinct expression domains found adjacent to each other. Our previous study [8] has shown that gbx1 expression initially overlaps with otx2 and then, during gastrulation, refines such that it sharply abuts the otx2 domain. Second, in the present work, we show that loss of gbx1 does not affect the initial posterior limit of otx2 at 60% epiboly, although gbx1 is rapidly required to maintain this boundary at 70% epiboly. In mice, it has been shown that the Otx2 and Gbx2 domains are initially established independently of each other at early headfold stage (E7.75), but that their expression becomes rapidly interdependent by late headfold stage [17,18,55]. Also in chicken, Garda et al. [56] described a phase when Otx2 and Gbx2 overlap in the mid-hindbrain neuroectoderm. This co-expression disappears at HH stage 14–15 and both domains become mutually exclusive and complementary [56]. Third, in mice, misexpression of Otx2 and Gbx2 during later segmentation stages can shift the MHB organizer [20,57]. We have shown here that raising the dosage of gbx1 shifts MHB position, mimicking the mouse Gbx2 gain-of-function experiments [57]. Fourth, we also show that ectopic juxtaposition of otx2 and gbx1 acts to induce MHB markers in the same way as ectopic juxtapositon of Otx2 and Gbx2 does in the mouse [21,23-25]).
Together with our previous work [8], the present study here illustrates that functional requirements for MHB formation may be achieved differently in zebrafish as compared to other species. Namely, gbx1, instead of gbx2, may be required early in zebrafish development for the correct specification of the MHB primordium. In zebrafish, it has been shown that gbx2 is expressed only at the end of gastrulation, after the MHB is established, in response to Fgf8 signaling [8]. Given that gbx2 has the same ability as gbx1 to suppress otx2 expression in the fore-midbrain region (data not shown) [9], it is possible that positioning of the zebrafish MHB through otx2 repression is later reinforced by the expression of a second gbx gene. Indeed, the gbx1 expression domain begins to fade from the MHB around the five to six somite stage [8]. It is possible that gbx2 steps in at this time to maintain the regulatory loops already in place. This is different from the situation in mouse, where only one Gbx gene, namely Gbx2, is expressed at the MHB and is maintained there during development [30,31].
gbx1 is a posteriorizing factor
Previous work in fish has shown that neural posteriorization is mediated via signals from the marginal blastoderm, the non-axial mesendoderm of pre-gastrula stage embryos [58]. Transplantation of cells from this region into the animal pole of the embryo has previously been shown to induce posterior neural markers, krox20, hoxa1 or gbx1, in the host tissue, suggesting that secreted molecules might be involved in this process [32,58,59]. Wnts, Nodals, retinoic acid and fibroblast growth factors are all good candidates to be posteriorizing molecules (for a review, see [60]). As of yet, experiments addressing this early neural posteriorization have only addressed the function of secreted factors; the role of transcription factors is unexplored. We have recently shown that Wnt8, a known posteriorizing molecule, is required for the initial subdivision of the neuroectoderm, including for the onset of posterior gbx1 expression [32]. Here we have shown that gbx1 overexpression, like wnt8 overexpression, posteriorizes the neuroectoderm in a cell-autonomous fashion and that gbx1 overexpression can partially restore hindbrain patterning in the absence of Wnt8. Collectively, our data point to a role for this homeodomain transcription factor in mediating Wnt posteriorizing activity during neuroectoderm development. In this process it likely cooperates with other transcription factors expressed in the margin and which mediate Wnt signaling during gastrulation. Two Sp1-related transcription factors, sp5 and sp5-like, are known to be direct targets of Wnt signaling and to mediate hindbrain patterning [49]. Furthermore, two caudal-related genes, cdx1a and cdx4, are expressed in the blastoderm margin during early gastrulation [61] and are regulated by the Wnt/β-catenin pathway [62]. It thus seems likely that the gradient of Wnt activity subdivides the posterior neural plate by activating specific transcription factors at different positions. These subdomains are established at the end of gastrulation (80% epiboly), a stage when hindbrain/spinal cord progenitors are proposed to acquire regional identity [63].
Materials and methods
Whole-mount in situ hybridization and antibody staining
ISH and antibody detection were performed using protocols described in [39]. Probes and wild-type expression patterns were as previously described for: krox20 [64]; otx2 [65]; pax2.1 [66]; eng2 [41,42]; wnt1 [40]; fgf8 [39]; gbx1 and gbx2 [8]; myoD [67]; six3 [68]; emx1 [37]; pax6 [69]; hoxa1 [43]; cdx4/caudal [45]; val [44]; and hoxb1 [70]. Antibody staining against acetylated tubulin (Sigma, St Louis, MO, USA)) was carried out as previously described [71].
Transplantation
Donor embryos were injected with biotin coupled tetramethyl-rhodamine dextran (10,000 MW, Molecular Probes D-1817, Eugene, OR, USA) diluted in 0.25 M KCl. Transplantation of donor cells into host embryos was done at shield stage using trimmed borosilicate capillaries. Transplanted cells were then visualized by immunochemical staining using the Vectastain ABC system (VectorLabs, Burlingame, CA, UK)) and DAB (Sigma).
Labeling of cell clones via laser-based activation of caged fluorescein
Non-fluorescent, photoactivatable (caged) fluorescein was used as a cell tracer for fate mapping in the zebrafish embryo as previously described [72].
DNA constructs
cDNAs encoding gbx1 or otx2 were subcloned into the pCS2+ or pCS2+MT vectors [73].
RNA and morpholino injection
Capped mRNAs were synthesized as previously described [39]. For RNA injection, embryos were dechorionated using pronase and injected at the one-cell stage. Two antisense morpholino oligonucleotides (Gene-Tools, Inc., Philomath, Oregon, USA) were designed to target gbx1 (Mo1 and Mo2): Mo1, 5' AAATCCCGTGCTGTACTGGCCTTCA 3'; Mo2, 5' CGCTGCTGAAGGGTCCTCGCCGTCC 3'. Morpholinos were resuspended in sterile water, stored at -20°C as 10 mg/ml solutions and diluted before use to the appropriate concentration in water containing 0.2% phenol red. Morpholinos were injected in the yolk with a concentration of 1–10 ng between the one- to four-cell stage.
Western blotting
For western blot analysis, embryos were deyolked and proteins were extracted from injected or non-injected embryos. Protein extracts were resolved by standard SDS-PAGE. Samples were electroblotted onto Protan nitrocellulose (Schleicher and Schuell, Dassel, Germany)). Membranes were incubated in Tris-buffered saline 1% low-fat milk for 1 h at room temperature with the anti-c-myc antibody (9E10) or E7 anti-tubulin ascites (Developmental Studies Hybridoma Bank). Immunocomplexes were revealed by chemiluminescence (Amersham, Buckinghamshire, UK)) with anti-mouse or anti-rabbit immunoglobin G antibodies conjugated to peroxidase (Sigma). Maintenance of and experimentation with zebrafish is covered under permits 24D-9165.40/1-2007 and 24D-9168.11-1/2008-3, and for genetic engineering work under permit 56-8811.71/189 of the State Government of Saxony, Germany, to MB.
Abbreviations
A-P: antero-posterior; ISH: in situ hybridization; MHB: midbrain-hindbrain boundary; Mo: morpholino; rh: rhombomere.
Authors' contributions
MR conceived the studies, designed and carried out the experiments, analyzed and interpreted the data, and wrote the article. KL performed injection experiments, cell-tracing experiments and analyzed data. RA helped with the injection experiments. MW helped with the ISH. MB obtained the funding, conceived the study, designed the research, analyzed data and wrote the article.
Supplementary Material
Comparison of gbx1 Mo1 and Mo2 induced phenotypes. (A-F) Lateral views, anterior to the top (the tailbud stage) and to the left (24 h). (G-K') Dorsal views, anterior to the top. (A) Control embryo at the tailbud stage; (B, B') embryos at the tailbud stage injected with 5 ng and 10 ng Mo1 respectively; (C, C') embryos at the tailbud stage injected with 5 ng and 10 ng Mo2 respectively. (D) Control embryo at 24 h; (E) 24 h embryo injected with 5 ng Mo1; (F) 24 h embryo injected with 5 ng Mo2.
krox20 expression at somite stage in gbx1 morphants. (A) Control embryo at the seven-somite stage stained with otx2/krox20/myoD. otx2 is not seen in this picture. The most anterior expression seen is the krox20 domain. myoD indicates the number of somites. (B) Embryos at the seven-somite stage injected with 7 ng of Mo1. The most anterior expression seen is the border of the otx2 domain and krox20 is not visible.
Acknowledgments
Acknowledgements
We thank all past and present members of the Brand lab for support and constructive discussions; Matthias Nowak, Arndt Siekmann and Laurel Rhode for critical reading of the manuscript; Pascal Dollé for discussions and insightful comments, and Claudia Lohs and Daniela Röllig for taking part in this project. We also thank Marika Fischer and Katrin Sippel for excellent fish maintenance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 655 and Br 1746-1-3) and the EU (ZF Models and Endotrack).
Contributor Information
Muriel Rhinn, Email: rhinn@igbmc.fr.
Klaus Lun, Email: Klaus.Lun@leica-microsystems.com.
Reiner Ahrendt, Email: reiner.ahrendt@biotec.tu-dresden.de.
Michaela Geffarth, Email: michaela.geffarth@biotec.tu-dresden.de.
Michael Brand, Email: michael.brand@biotec.tu-dresden.de.
References
- Meinhardt H. Cell determination boundaries as organizing regions for secondary embryonic fields. Dev Biol. 1983;96:375–385. doi: 10.1016/0012-1606(83)90175-6. [DOI] [PubMed] [Google Scholar]
- Joyner AL. Engrailed, Wnt and Pax genes regulate midbrain-hindbrain development. Trends Genet. 1996;12:15–20. doi: 10.1016/0168-9525(96)81383-7. [DOI] [PubMed] [Google Scholar]
- Wassef M, Joyner AL. Early mesencephalon/metencephalon patterning and development of the cerebellum. Perspect Dev Neurobiol. 1997;5:3–16. [PubMed] [Google Scholar]
- Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D'Apice M, Nigro V, Boncinelli E. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A, Brulet P. Forebrain and midbrain regions are deleted in Otx2-/- mutants due to a defective anterior neuroectoderm specification during gastrulation. Development. 1995;121:3279–3290. doi: 10.1242/dev.121.10.3279. [DOI] [PubMed] [Google Scholar]
- Ang SL, Jin O, Rhinn M, Daigle N, Stevenson L, Rossant J. A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development. 1996;122:243–252. doi: 10.1242/dev.122.1.243. [DOI] [PubMed] [Google Scholar]
- Wassarman KM, Lewandoski M, Campbell K, Joyner AL, Rubenstein JL, Martinez S, Martin GR. Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development. 1997;124:2923–2934. doi: 10.1242/dev.124.15.2923. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Lun K, Amores A, Yan YL, Postlethwait JH, Brand M. Cloning, expression and relationship of zebrafish gbx1 and gbx2 genes to Fgf signaling. Mech Dev. 2003;120:919–936. doi: 10.1016/S0925-4773(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Kikuta H, Kanai M, Ito Y, Yamasu K. gbx2 Homeobox gene is required for the maintenance of the isthmic region in the zebrafish embryonic brain. Dev Dyn. 2003;228:433–450. doi: 10.1002/dvdy.10409. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Brand M. The midbrain-hindbrain boundary organizer. Curr Opin Neurobiol. 2001;11:34–42. doi: 10.1016/S0959-4388(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- Canning CA, Lee L, Irving C, Mason I, Jones CM. Sustained interactive Wnt and FGF signaling is required to maintain isthmic identity. Dev Biol. 2007;305:276–286. doi: 10.1016/j.ydbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Lun K, Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–3062. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- Raible F, Brand M. Divide et impera – the midbrain-hindbrain boundary and its organizer. Trends Neurosci. 2004;27:727–735. doi: 10.1016/j.tins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dierich A, Shawlot W, Behringer RR, Le Meur M, Ang SL. Sequential roles for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction and specification. Development. 1998;125:845–856. doi: 10.1242/dev.125.5.845. [DOI] [PubMed] [Google Scholar]
- Acampora D, Avantaggiato V, Tuorto F, Briata P, Corte G, Simeone A. Visceral endoderm-restricted translation of Otx1 mediates recovery of Otx2 requirements for specification of anterior neural plate and normal gastrulation. Development. 1998;125:5091–5104. doi: 10.1242/dev.125.24.5091. [DOI] [PubMed] [Google Scholar]
- Millet S, Campbell K, Epstein D, Losos K, Harris E, Joyner A. A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature. 1999;401:161–164. doi: 10.1038/43664. [DOI] [PubMed] [Google Scholar]
- Martinez-Barbera JP, Signore M, Boyl PP, Puelles E, Acampora D, Gogoi R, Schubert F, Lumsden A, Simeone A. Regionalisation of anterior neuroectoderm and its competence in responding to forebrain and midbrain inducing activities depend on mutual antagonism between OTX2 and GBX2. Development. 2001;128:4789–4800. doi: 10.1242/dev.128.23.4789. [DOI] [PubMed] [Google Scholar]
- Li JYH, Joyner A. Otx2 and Gbx2 are required for refinement and not induction of mid-hindbrain gene expression. Development. 2001;128:4979–4991. doi: 10.1242/dev.128.24.4979. [DOI] [PubMed] [Google Scholar]
- Broccoli V, Boncinelli E, Wurst W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature. 1999;401:164–168. doi: 10.1038/43670. [DOI] [PubMed] [Google Scholar]
- Tour E, Pillemer G, Gruenbaum Y, Fainsod A. Gbx2 interacts with Otx2 and patterns the antero-posterior axis during gastrulation in Xenopus. Mech Dev. 2002;112:141–154. doi: 10.1016/S0925-4773(01)00653-0. [DOI] [PubMed] [Google Scholar]
- Tour E, Pillemer G, Gruenbaum Y, Fainsod A. Otx2 can activate the isthmic organizer genetic network in the Xenopus embryo. Mech Dev. 2002;110:3–13. doi: 10.1016/S0925-4773(01)00591-3. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Sanchez M, Simeone A, Alvarado-Mallart RM. Fgf8 and Gbx2 induction concomitant with Otx2 repression is correlated with midbrain-hindbrain fate of caudal prosencephalon. Development. 1999;126:3191–3203. doi: 10.1242/dev.126.14.3191. [DOI] [PubMed] [Google Scholar]
- Irving C, Mason I. Regeneration of isthmic tissue is the result of a specific and direct interaction between rhombomere 1 and midbrain. Development. 1999;126:3981–3989. doi: 10.1242/dev.126.18.3981. [DOI] [PubMed] [Google Scholar]
- Katahira T, Sato T, Sugiyama S, Okafuji T, Araki I, Funahashi J, Nakamura H. Interaction between Otx2 and Gbx2 defines the organizing center for the optic tectum. Mech Dev. 2000;91:43–52. doi: 10.1016/S0925-4773(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Simeone A. Positioning the isthmic organizer where Otx2 and Gbx2 meet. Trends Genet. 2000;16:237–240. doi: 10.1016/S0168-9525(00)02000-X. [DOI] [PubMed] [Google Scholar]
- Joyner AL, Liu A, Millet S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol. 2000;12:736–741. doi: 10.1016/S0955-0674(00)00161-7. [DOI] [PubMed] [Google Scholar]
- Liu A, Joyner AL. Early anterior/posterior patterning of the midbrain and cerebellum. Annu Rev Neurosci. 2001;24:869–896. doi: 10.1146/annurev.neuro.24.1.869. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Chazaud C, Oulad-Abdelghani M, Dolle P, Chambon P. Sequence and expression pattern of the Stra7 (Gbx-2) homeobox-containing gene induced by retinoic acid in P19 embryonal carcinoma cells. Dev Dyn. 1995;204:372–382. doi: 10.1002/aja.1002040404. [DOI] [PubMed] [Google Scholar]
- Waters ST, Wilson CP, Lewandoski M. Cloning and embryonic analysis of the mouse Gbx1 gene. Gene Expr Patterns. 2003;3:313–317. doi: 10.1016/S1567-133X(03)00041-3. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Lun K, Werner M, Simeone A, Brand M. Isolation and expression of the homeobox gene Gbx1 during mouse development. Dev Dyn. 2004;229:334–339. doi: 10.1002/dvdy.10435. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Lun K, Luz M, Werner M, Brand M. Positioning of the midbrain-hindbrain boundary organizer through global posteriorization of the neuroectoderm mediated by Wnt8 signaling. Development. 2005;132:1261–1272. doi: 10.1242/dev.01685. [DOI] [PubMed] [Google Scholar]
- Kozlowski DJ, Murakami T, Ho RK, Weinberg ES. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochem Cell Biol. 1997;75:551–562. doi: 10.1139/bcb-75-5-551. [DOI] [PubMed] [Google Scholar]
- Woo K, Fraser SE. Order and coherence in the fate map of the zebrafish nervous system. Development. 1995;121:2595–609. doi: 10.1242/dev.121.8.2595. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dierich A, Le Meur M, Ang S. Cell autonomous and non-cell autonomous functions of Otx2 in patterning the rostral brain. Development. 1999;126:4295–4304. doi: 10.1242/dev.126.19.4295. [DOI] [PubMed] [Google Scholar]
- Bellipanni G, Murakami T, Doerre O, Andermann P, Weinberg E. Expression of Otx homeodomain proteins induces cell aggregation in developing zebrafish embryos. Dev Biol. 2000;223:339–353. doi: 10.1006/dbio.2000.9771. [DOI] [PubMed] [Google Scholar]
- Morita T, Nitta H, Kiyama Y, Mori H, Mishina M. Differential expression of two zebrafish emx homeoprotein mRNAs in the developing brain. Neurosci Lett. 1995;198:131–134. doi: 10.1016/0304-3940(95)11988-9. [DOI] [PubMed] [Google Scholar]
- Reim G, Brand M. spiel-ohne-grenzen/pou2 mediates regional competence to respond to Fgf8 during zebrafish early neural development. Development. 2002;129:917–933. doi: 10.1242/dev.129.4.917. [DOI] [PubMed] [Google Scholar]
- Reifers F, Böhli H, Walsh EC, Crossley PH, Stainier DYR, Brand M. Fgf8 is mutated in zebrafish acerebellar mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Kelly GM, Lai CJ, Moon RT. Expression of wnt10a in the central nervous system of developing zebrafish. Dev Biol. 1993;158:113–121. doi: 10.1006/dbio.1993.1172. [DOI] [PubMed] [Google Scholar]
- Ekker M, Wegner J, Akimenko MA, Westerfield M. Coordinate embryonic expression of three zebrafish engrailed genes. Development. 1992;116:1001–1010. doi: 10.1242/dev.116.4.1001. [DOI] [PubMed] [Google Scholar]
- Fjose A, Eiken HG, Njolstad PR, Molven A, Hordvik I. A zebrafish engrailed-like homeobox sequence expressed during embryogenesis. FEBS Lett. 1988;231:355–360. doi: 10.1016/0014-5793(88)80849-4. [DOI] [PubMed] [Google Scholar]
- Alexandre D, Clarke DJ, Oxtoby E, Yan YL, Jowett T, holder N. Ectopic expression of Hoxa-1 in the zebrafish alters the fate of the mandibular arch neural crest and phenocopies a retinoic acid-induced phenotype. Development. 1996;122:735–746. doi: 10.1242/dev.122.3.735. [DOI] [PubMed] [Google Scholar]
- Moens C, Yan YL, Appel B, Force A, Kimmel C. valentino: a zebrafish gene required for normal hindbrain segmentation. Development. 1996;122:3981–3990. doi: 10.1242/dev.122.12.3981. [DOI] [PubMed] [Google Scholar]
- Joly JS, Maury M, Joly C, Duprey P, Boulekbache H, Condamine H. Expression of a zebrafish caudal homeobox gene correlates with the establishment of posterior cell lineages at gastrulation. Differentiation. 1992;50:75–87. doi: 10.1111/j.1432-0436.1992.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development. 1995;121:1787–1799. doi: 10.1242/dev.121.6.1787. [DOI] [PubMed] [Google Scholar]
- Erter CE, Wilm TP, Basler N, Wright CV, Solnica-Krezel L. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 2001;128:3571–3583. doi: 10.1242/dev.128.18.3571. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/S1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Martinez S, Wassef M, Alvarado-Mallart RM. Induction of a mesencephalic phenotype in the 2-day-old chick prosencephalon is preceded by the early expression of the homeobox gene en. Neuron. 1991;6:971–981. doi: 10.1016/0896-6273(91)90237-T. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Buckles GR, Kostakis N, Moon RT. Wnt1 and wnt10b function redundantly at the zebrafish midbrain-hindbrain boundary. Dev Biol. 2003;254:172–187. doi: 10.1016/S0012-1606(02)00044-1. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Takemaru K, Bates R, Moon RT. Direct regulation of the Xenopus engrailed-2 promoter by the Wnt signaling pathway, and a molecular screen for Wnt-responsive genes, confirm a role for Wnt signaling during neural patterning in Xenopus. Mech Dev. 1999;87:21–32. doi: 10.1016/S0925-4773(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Liu A, Joyner AL. EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development. 2001;128:181–191. doi: 10.1242/dev.128.2.181. [DOI] [PubMed] [Google Scholar]
- Garda AL, Echevarria D, Martinez S. Neuroepithelial co-expression of Gbx2 and Otx2 precedes Fgf8 expression in the isthmic organizer. Mech Dev. 2001;101:111–118. doi: 10.1016/S0925-4773(00)00567-0. [DOI] [PubMed] [Google Scholar]
- Millet S, Bloch-Gallego E, Simeone A, Alvarado-Mallart RM. The caudal limit of Otx2 gene expression as a marker of the midbrain/hindbrain boundary: a study using in situ hybridisation and chick/quail homotopic grafts. Development. 1996;122:3785–3797. doi: 10.1242/dev.122.12.3785. [DOI] [PubMed] [Google Scholar]
- Woo K, Fraser SE. Specification of the zebrafish nervous system by nonaxial signals. Science. 1997;277:254–257. doi: 10.1126/science.277.5323.254. [DOI] [PubMed] [Google Scholar]
- Momoi A, Yoda H, Steinbeisser H, Fagotto F, Kondoh H, Kudo A, Driever W, Furutani-Seiki M. Analysis of Wnt8 for neural posteriorizing factor by identifying Frizzled 8c and Frizzled 9 as functional receptors for Wnt8. Mech Dev. 2003;120:477–489. doi: 10.1016/S0925-4773(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Sive H. Early anteroposterior division of the presumptive neurectoderm in Xenopus. Mech Dev. 2001;104:21–36. doi: 10.1016/S0925-4773(01)00358-6. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Ernst P, Wang Y, Dekens MP, Kingsley PD, Palis J, Korsmeyer SJ, Daley GQ, Zon LI. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Woo K, Fraser SE. Specification of the hindbrain fate in the zebrafish. Dev Biol. 1998;197:283–296. doi: 10.1006/dbio.1998.8870. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier P, Simeone A, Cotelli F, Boncinelli E. Expression pattern of two otx genes suggests a role in specifying anterior body structures in zebrafish. Int J Dev Biol. 1995;39:559–573. [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Fjose A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development. 1991;113:1193–1206. doi: 10.1242/dev.113.4.1193. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- Seo HC, Drivenes , Ellingsen S, Fjose A. Expression of two zebrafish homologues of the murine Six3 gene demarcates the initial eye primordia. Mech Dev. 1998;73:45–57. doi: 10.1016/S0925-4773(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Xu Q, Barth KA, Mikkola I, Holder N, Fjose A, Krauss S, Wilson SW. Regulatory gene expression boundaries demarcate sites of neuronal differentiation in the embryonic zebrafish forebrain. Neuron. 1994;13:1039–1053. doi: 10.1016/0896-6273(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Prince VE, Moens CB, Kimmel CB, Ho RK. Zebrafish hox genes: expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development. 1998;125:393–406. doi: 10.1242/dev.125.3.393. [DOI] [PubMed] [Google Scholar]
- Shanmugalingam S, Houart C, Picker A, Reifers F, MacDonald R, Barth AK, Brand M, Wilson SW. Ace/Fgf8 is required for forebrain commissure formation and patterning of the telencephalon. Development. 2000;127:2549–2561. doi: 10.1242/dev.127.12.2549. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Lohs C, Brand M. Engrailed and Fgf8 act synergistically to maintain the boundary between forebrain and midbrain. Development. 2003;130:4881–4893. doi: 10.1242/dev.00683. [DOI] [PubMed] [Google Scholar]
- Rupp RAW, Snider L, Weintraub H. Xenopus embryos regulatie the nuclear localization of XMyoD. Genes Dev. 1994;8:1311–1323. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of gbx1 Mo1 and Mo2 induced phenotypes. (A-F) Lateral views, anterior to the top (the tailbud stage) and to the left (24 h). (G-K') Dorsal views, anterior to the top. (A) Control embryo at the tailbud stage; (B, B') embryos at the tailbud stage injected with 5 ng and 10 ng Mo1 respectively; (C, C') embryos at the tailbud stage injected with 5 ng and 10 ng Mo2 respectively. (D) Control embryo at 24 h; (E) 24 h embryo injected with 5 ng Mo1; (F) 24 h embryo injected with 5 ng Mo2.
krox20 expression at somite stage in gbx1 morphants. (A) Control embryo at the seven-somite stage stained with otx2/krox20/myoD. otx2 is not seen in this picture. The most anterior expression seen is the krox20 domain. myoD indicates the number of somites. (B) Embryos at the seven-somite stage injected with 7 ng of Mo1. The most anterior expression seen is the border of the otx2 domain and krox20 is not visible.