Abstract
Background and Purpose
Deferoxamine (DFX) reduces brain edema, neuronal death and neurological deficits after intracerebral hemorrhage (ICH) in young rats. In the present study, we investigated whether DFX is effective on brain injury after ICH in aged rats and examined dose dependency.
Methods
Male Fischer 344 rats (18-months old) had an intracaudate injection of 100-µL autologous whole blood and were treated with different doses of DFX (10, 50 and 100 mg/kg) or vehicle 2 and 6 hours post ICH and then every 12 h up to 7 days. Rats were sacrificed at day 3 for brain edema determination and day 56 for brain atrophy measurement. Behavioral tests were performed during the experiments.
Results
All three doses of DFX attenuated perihematomal brain edema at 3 days (e.g. at dose 50 mg/kg, 80.4±0.5 vs. 81.6±0.9% in the vehicle-treated group, p<0.01). 50 and 100 mg/kg DFX also reduced ICH-induced ventricle enlargement, caudate atrophy and ICH-induced neurological deficits in aged rats. However, while 10 mg/kg DFX reduced ventricle enlargement and forelimb placing deficits, it did not reduce caudate atrophy and corner turn deficits.
Conclusions
These results indicate that DFX can reduce ICH-induced brain injury in aged as well as young rats and that a dose higher than 10 mg/kg is the optimal dose of DFX in this model.
Keywords: brain atrophy, cerebral hemorrhage, deferoxamine, iron
Introduction
Intracerebral hemorrhage (ICH) is a common and often fatal stroke subtype. Many patients with an intracerebral hematoma experience a progressive deterioration in their neurological condition due to formation of secondary brain edema1, 2. Even if the patients survive this acute phase, prolonged neurological deficits and brain atrophy commonly occur3–5.
Iron, a heme degradation product, has an important role in ICH-induced brain injury. After erythrocyte lysis, iron concentrations in the brain can reach very high levels. We have shown a 3-fold increase of brain non-heme iron after intracerebral hemorrhage in rats, and brain iron levels remain high for at least several weeks6. Deferoxamine (DFX), an iron chelator, can rapidly penetrate the blood-brain barrier and accumulate in the brain tissue at a significant concentration after systemic administration7, 8. Our previous studies have found that DFX reduces ICH or hemoglobin-induced brain edema, neuronal death, neurological deficits and brain atrophy in young rats3, 9–12.
ICH is mostly a disease of the elderly, but current experimental ICH models have primarily used young animals. Age is an important factor affecting brain injury in ischemic stroke in animals and humans13, 14. Recently, we found that ICH caused greater neurological deficits, more severe brain swelling, greater induction of heat shock proteins and enhanced microglial activation in aged rats compared to young rats15. Age is an important predictor of mortality following ICH in humans16. These results suggest that age is a significant factor in determining brain injury after ICH.
In relation to iron-induced brain injury after ICH, aged rats have higher levels of heme oxygenase-1 (HO-1) after ICH15. That enzyme is involved in the production of iron from heme during hematoma resolution and inhibitors of HO-1 have been shown to reduce ICH-induced brain injury17. In addition, increased microglial activation in aged rats15 may also impact iron-handling. Apart from being the major site of HO-1 expression after ICH, microglia also show a marked increase in L- and H-ferritin expression, major endogenous iron-chelators, after ICH6. These findings suggested that aging might impact the protective effects of DFX in ICH.
The present study, therefore, investigated whether DFX is effective on brain injury including brain edema, prolonged neurological deficits and brain atrophy after ICH in aged rats. It also examined the optimal dose for DFX to reduce ICH-injury.
Materials and Methods
Animal Preparation and Intracerebral Infusion
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of ninety-nine 18-month old male Fischer 344 rats (weight, 380 to 450 g; NIH) were used in this study. Rats were anesthetized with pentobarbital (45 mg/kg, i.p.). The right femoral artery was catheterized for continuous blood pressure monitoring and blood sampling. Blood was obtained from the catheter for analysis of blood pH, PaO2, PaCO2, hematocrit, and blood glucose. Core temperature was maintained at 37°C with use of a feedback-controlled heating pad. Rats were positioned in a stereotactic frame (Kopf Instruments), and a cranial burr hole (1 mm) was drilled on the right coronal suture 3.5 mm lateral to the midline. A 26-gauge needle was inserted stereotactically into the right basal ganglia (coordinates: 0.2 mm anterior, 5.5 mm ventral, 3.5 mm lateral to the bregma). Autologous whole blood (100 µL) was injected at a rate of 10 µL/minute using a microinfusion pump (Harvard Apparatus Inc.). Sham controls had only an intracerebral needle insertion. After injection, the needle was removed, the burr hole was filled with bone wax, and the skin incision was closed with sutures.
Experimental Groups
There were two sets of experiments in this study. In the first set, ICH rats were treated with DFX (10, 50 or 100 mg/kg administered intramuscularly; n=9 for each dose) or vehicle (n=14) 2 and 6 hours after ICH and then every 12 h for 3 days. Sham rats were treated with DFX 100 mg/kg (n=3) or vehicle (n=6) after surgery as ICH rats. Rats were sacrificed at day 3 for brain edema measurement. In the second set, rats were treated with DFX (10, 50 or 100 mg/kg; n=9 for each dose) or vehicle (n=12) 2 and 6 hours post ICH and then every 12 h for 7 days. Sham rats were treated with DFX 100 mg/kg (n=5) or vehicle (n=5). Rats had T2-weighted magnetic resonance images at eight weeks after ICH, and then they were sacrificed for histological analyses. Behavioral tests were undertaken on the day before surgery, and then 1, 28, 56 days after surgery. DFX administration was not blinded, but brain atrophy and neurological deficits were measured by an investigator who was blinded to the treatment status of the animal. Body weight and blood pressure were measured at 1, 3, 7, 14, 21, 28, 42 and 56 days after surgery. Body weight was expressed as % change in body weight using this formula: % change in body weight = (body weight on each time point-body weight before surgery)/body weight before surgery. Mean arterial blood pressure was measured using tail cuff plethysmography (IITC Life Science). The heat chamber was set at 29 °C for optimal tail arterial dilatation to allow the measurement of the pulsatile pressure. A tail cuff/sensor was inflated by the system to a maximum pressure of ~150 mmHg, and mean arterial blood pressure was determined using the optical sensor.
Brain Water and Ion Contents
Animals were anesthetized again and decapitated to measure brain water and ion contents. The rat brains were removed, and a coronal tissue slice (3-mm thick) 4 mm from the frontal pole was cut using a blade. The brain tissue slice was divided into two hemispheres along the midline, and each hemisphere was dissected into cortex and basal ganglia. The cerebellum served as a control. Five tissue samples from each brain were obtained: the ipsi- and contralateral cortex, the ipsi- and contralateral basal ganglia, and the cerebellum. Brain samples were immediately weighted on an electric analytical balance (model AE 100; Mettler Instrument) to obtain the wet weight. Brain samples were dried at 100°C for 24 hours to obtain the dry weight. The formula for our calculations was the following: (wet weight–dry weight)/wet weight. Then the dehydrated samples were digested in 1 ml of 1 mol/L nitric acid for 1 week. Sodium and potassium contents of this solution were measured using the automatic flame photometer (model IL 943; Instrumentation Laboratory). Ion content was expressed in microequivalents per gram of dehydrated brain tissue (mEq/kg dry wt)18.
Behavioral Tests
Intracerebral hemorrhage-induced neurological deficits were assessed using forelimb placing and corner turn tests4. In the vibrissae-elicited forelimb placing test, animals were held by their bodies to allow the forelimbs to hang free. Independent testing of each forelimb was conducted by brushing the respective vibrissae on the corner of a table top once per trial for 10 trials. A score of 1 was given each time the rat placed its forelimb onto the edge of the table in response to vibrissae stimulation. The percentage of successful placing responses was determined for the impaired and the unimpaired forelimbs.
For the corner turn test, the rat was allowed to proceed into a corner whose angle was 30°. To exit the corner, the animal could turn to either the left or the right, and the direction was recorded. This task was repeated 10 to 15 times, and the percentage of right turns calculated.
Magnetic Resonance Imaging (MRI)
Magnetic resonance scans were performed at 2 months after ICH. All rats were anesthetized with 1.5 to 2% isofluorane/air mixture throughout MRI examination. Rats lay prone, head first in a 7.0T Varian MR scanner (183-mm horizontal bore, Varian) with the body temperature maintained at 37°C, using circulated heated air. A double-tuned volume radiofrequency coil was used to scan the head region of the rats. Axial T2-weighted images were acquired using a fast spin-echo sequence with the following parameters: repetition time (TR)/effective echo time (TE), 4000/60 ms; field of view (FOV), 50×50 mm; matrix, 256×128; slice thickness, 1.0 mm; slice spacing, 0 mm; number of slices, 25; and number of scans, 1 (total scan time ~2 min.). Five MRI slices, from the slice showing the front tip of lateral ventricle to 5 mm posterior, were scanned on a computer. Then bilateral ventricles were outlined and outlined areas were measured using ImageJ (version 1.37v; National Institutes of Health). Ventricle volume was obtained by combining the five ventricle areas and multiplying by the thickness (1 mm) of the sections. All measurements were repeated three times and the mean value was used. Ventricle volume was expressed as a percentage of the ipsilateral/contralateral.
Brain Atrophy Measurement
Rats were again anesthetized (intraperitoneal pentobarbital 60 mg/kg) and underwent transcardiac perfusion with 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (pH 7.4). The brains were removed and kept in 4% paraformaldehyde for 6 hours, then immersed in 30% sucrose for 3 to 4 days at 4°C. Brains were then placed in optimal cutting temperature embedding compound (Sakura Finetek, Inc.) and sectioned on a cryostat (18-µm thick slices). We estimated brain atrophy morphometrically. Coronal sections from 1 mm posterior to the blood injection site were stained with hematoxylin and eosin (H & E), and they were scanned. The bilateral caudate were outlined on a computer, and caudate size measured as described in the MRI method. To minimize the influence of tissue shrinkage, brain atrophy was expressed as a percentage of the ipsilateral/contralateral area.
Statistical Analysis
All data in this study are presented as means ± SD. Data were analyzed with Student’s t-test or one-way analysis of variance (ANOVA). Differences were considered significant at p<0.05.
Results
Physiological Variables
All physiological variables were measured immediately before an intracerebral infusion. Mean arterial blood pressure, blood pH, PaO2, PaCO2, hematocrit, and blood glucose level were controlled within normal ranges (data not shown).
Brain Edema
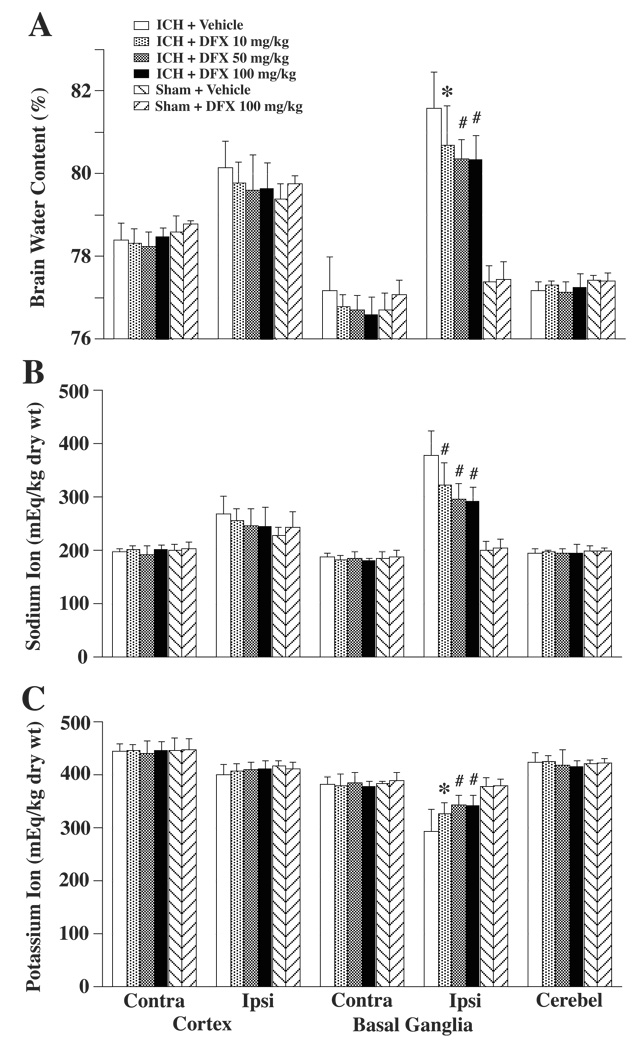
The effect of DFX treatment on brain edema formation in aged rats was assessed 3 days after induction of ICH. ICH caused a marked increase in perihematomal water content in vehicle-treated rats (81.6±0.9 vs. 77.7±0.4% in sham control, p<0.01). Systemic administration of 100 mg/kg DFX starting 2 hours after ICH reduced brain edema (80.3±0.6% vs. 81.6±0.9% in the vehicle-treated group; p<0.01). DFX at doses 50 and 10 mg/kg also reduced perihematomal brain edema (80.4±0.5%, p<0.01, and 80.7±0.9%, p<0.05, respectively; Figure 1A). The amelioration of ICH-induced edema formation with DFX was associated with a reduced accumulation of sodium and a reduced loss of potassium in the ipsilateral basal ganglia (Figure 1B and Figure 1C). DFX treatment did not change brain water and ion contents in sham-operated rats (Figure 1).
Figure 1.
Bar graph showing brain water (A), sodium (B) and potassium (C) content at 3 days after ICH. Values are expressed as the means ± SD. Contra = contralateral, Ipsi = ipsilateral, Cerebel = cerebellum. *p<0.05, #p<0.01 vs. ICH + Vehicle group.
Body Weight, Mean Arterial Blood Pressure and Mortality
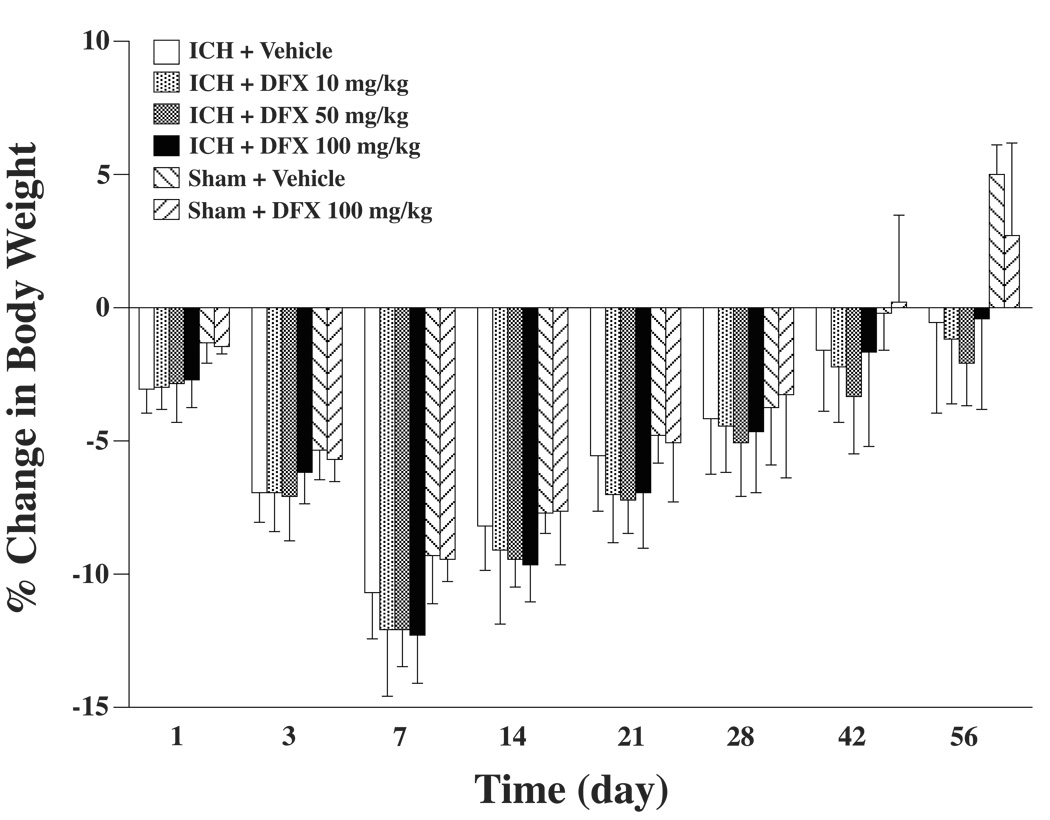
During the long-term experiments, body weight and mean arterial blood pressure were monitored. Rat body weight was decreased after surgery and the peak of body weight reduction was at day 7. From that point, rat body weight gradually increased. DFX treatment did not affect the body weight change among the ICH groups. Also, in sham rats, there were no differences between DFX- and vehicle-treated groups (Figure 2).
Figure 2.
Bar graph displaying body weight reduction during the experiment. Values are expressed as the means ± SD.
As measured by tail cuff plethysmography, mean arterial blood pressure did not differ significantly during the time course of the experiments for any group (group mean values varies from 109 to 114 mmHg). Nor was it different between the experimental groups. Thus, there were no differences in blood pressure with DFX treatment in either the ICH or sham-operated rats, nor were there differences between ICH and sham-operated rats.
Mortality rate in ICH rats was low in this study. There was one death at day 33 in the vehicle treated group, one death at day 4 in the DFX 10 mg/kg group and one death at day 49 in the DFX 50 mg/kg group.
Neurological Deficits
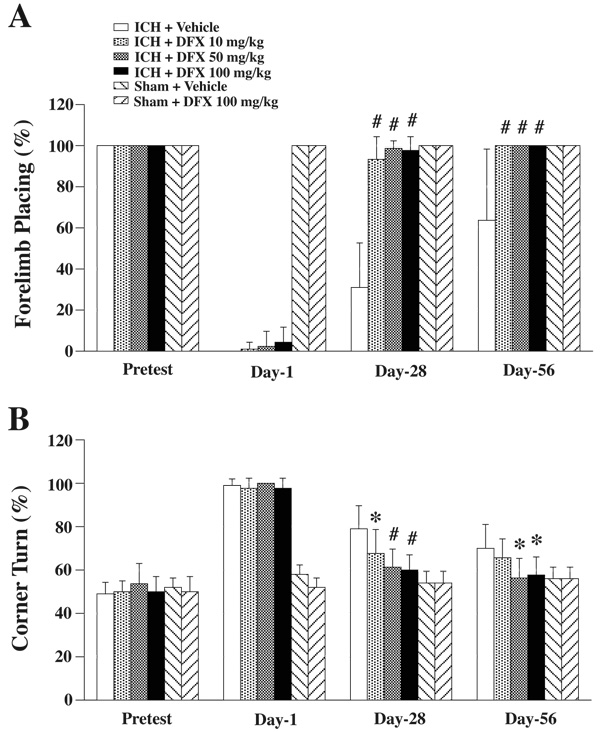
Behavioral tests including the forelimb placing and the corner turn tests were performed before ICH, and 1, 28 and 56 days after ICH. In vehicle-treated ICH rats, a partial recovery of forelimb placing occurred with time, but residual neurological deficits were still present at 56 days (64±35% response rate). When rats were treated with DFX, ICH-induced forelimb placing deficits showed a greater recovery (over 90% response rate at 28 and 56 days; Figure 3A). In the corner turn test, the percentage of turns to the right was significantly decreased at 28 days in ICH + DFX 10 mg/kg (68±11%, p<0.05), 50 mg/kg (61±8%, p<0.01) and 100 mg/kg (60±7%, p<0.01) treatment groups compared to ICH + vehicle group (79±10%). ICH-induced corner turn deficits were almost normalized (equal turns to right and left) at 56 days in DFX 50 mg/kg (56±9% right turns) and 100 mg/kg (58±8% right turns) treatment groups (Figure 3B). DFX 100 mg/kg treatment did not cause any neurological deficits in sham rats (Figure 3A and B).
Figure 3.
Forelimb placing (A) and corner turn (B) test scores prior to ICH, and 1, 28, 56 days after ICH. Values are expressed as the means ± SD. *p<0.05, #p<0.01 vs. ICH + Vehicle group.
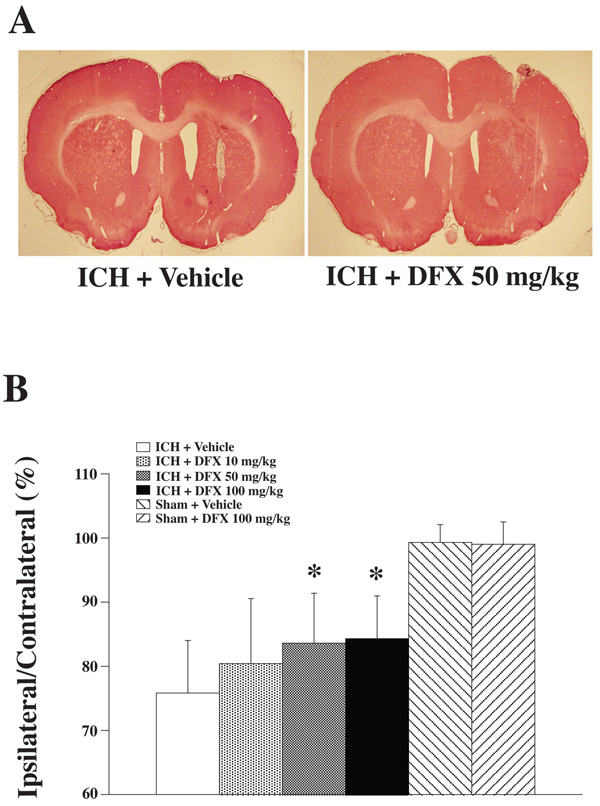
Brain Atrophy
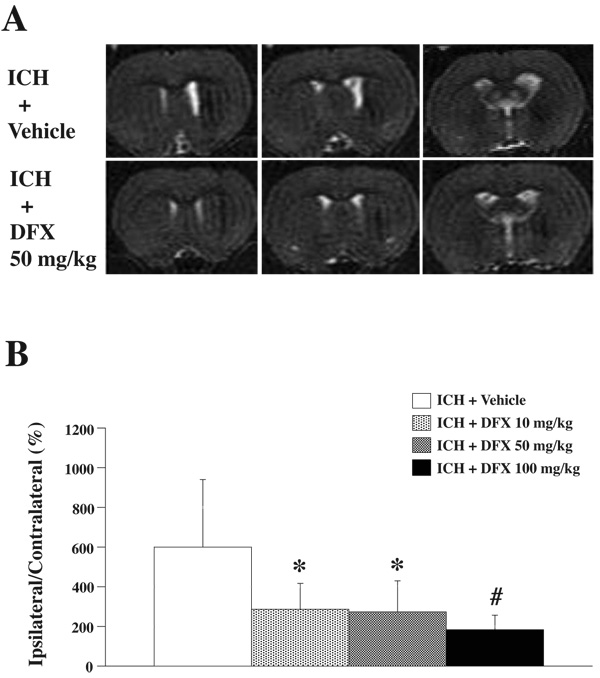
Rats had T2-weighted MR imaging at 2 months after ICH (Figure 4A). MRI showed the ipsilateral ventricle enlargement induced by ICH (600±339% in ICH + vehicle group). DFX reduced ICH-induced ventricle volume enlargement (Figure 4B). Ipsilateral caudate atrophy as compared to the contralateral side was found in coronal brain sections stained with H & E at 2 months after ICH (Figure 5A). In vehicle-treated rats, the ipsilateral caudate was 75.8±8.3% of contralateral. DFX treatment with 50 mg/kg or 100 mg/kg significantly reduced caudate atrophy (83.6±7.8 and 84.3±6.7% of contralateral; p<0.05 vs vehicle treated ICH group). There was a tendency for a reduction in caudate atrophy in the 10 mg/kg group, but this did not reach significance (Figure 5B).
Figure 4.
A: T2-weighted magnetic resonance images at 2 months after ICH treated with vehicle or DFX 50 mg/kg. B: Bar graph demonstrating the ventricle volume expressed as a percentage of the contralateral side. Values are expressed as the means ± SD. *p<0.05, #p<0.01 vs. ICH + Vehicle group.
Figure 5.
A: Coronal gross H&E sections eight weeks after ICH treated with vehicle or DFX 50 mg/kg. B: Bar graph demonstrating caudate size expressed as a percentage of the contralateral side. Values are expressed as the means ± SD. *p<0.05, #p<0.01 vs. ICH + Vehicle group.
Discussion
In the present study, we found that DFX treatment attenuated ICH-induced brain edema, neurological deficits and brain atrophy in aged rats without affecting body weight and mean arterial blood pressure. These results suggest that iron chelation may be a useful therapy for ICH patients.
Aged Fischer rats (18-month old), which were commercially available from the National Institute on Aging, were used. The average lifespan of man is 72 years and the average life span of a male rat is between 2 and 3 years. As a % of average life span, 18-month old in a rat corresponds to 50-year old in a human.
Based on the clinical experience with tissue plasminogen activator for acute ischemic stroke, we believed that 2 hours post ictus is the earliest time point that DFX could be administered for ICH. Our results indicate that iron contributes to acute as well as delayed brain injury10. In the acute phase, iron can potentiate thrombin toxicity in the brain19. Given those results, starting DFX treatment at 2 hours would likely maximize induced protection.
The three major endpoints in our study were brain edema, brain atrophy and neurological scores. Perihematomal edema is thought by many, but not all, to be a major cause of death and disability following ICH, particularly in relation to herniation1, 20–22. In combination with the presence of the hematoma, further mass effect due to edema formation can result in a midline shift and herniation. Perihematomal edema, as seen on CT scan, can result from clot retraction. However, that represents a redistribution of fluid between hematoma and brain. A progressive mass effect can only result from a movement of fluid from the blood to brain, either in the form of hematoma enlargement and/or progressive perihematomal edema. Although all agree that the latter does occur, its extent/prevalence has been debated22. It is easy to examine where perihematomal tissue can be sampled and water content determined directly in animal models. In animals there is substantial evidence for progressive edema formation18, 23–26.
Our studies on ICH have indicated that thrombin and iron are two major factors that responsible for brain edema formation1, 27. Perihematomal brain edema is maximal at day 3 in rats. We, therefore, tested the effects of deferoxamine on ICH-induced edema at day 3. The degree of protection found with 100 mg/kg DFX, a reduction in water content from 81.6 to 80.3%, was very similar to that found in young rats (81.2 to 79.9%;15).
Brain atrophy has been found in animals and humans after ICH28–30. The underlying cause(s) of this atrophy is, however, unknown. Our recent study suggested iron overload is associated with brain atrophy after ICH and deferoxamine reduces ICH-induced brain atrophy in young rats3. Here we demonstrate that iron contributes to brain atrophy development after ICH in aged rats.
We found that DFX reduced ICH-induced neurological deficits in aged rats at 4 and 8 weeks. All two sensorimotor behavioral tests appear to be well suited to models of unilateral brain injury because they measure asymmetry. Thus, they can factor out confounding variables for behavioral tests such as decreased overall activity following surgery. These sensorimotor tests are also not altered by repeated testing and they do not require special training or food deprivation31. The degree of improvement in neurological deficits with 100 mg/kg DFX in aged rats was similar to that we have found previously with young rats where there was almost a complete normalization of forelimb placing and corner test scores in DFX treated animals by 28 days3.
DFX (10, 50 or 100 mg/kg) were given intramuscularly as suggested by Novartis Pharmaceuticals Corp. The maximal dose, 100 mg/kg, was chosen based on our previous studies which indicated this dose was effective in reducing ICH-induced brain injury3, 10. The lower doses were chosen based on normal dosing in humans for other conditions. Deferoxamine is normally given as a 1000 mg dose followed by 500 mg every 4 to 12 hours if needed. The maximal recommended daily dose is 6000 mg. Assuming a body weight of 80 kg, the initial human dose would be 12.5 mg/kg, hence our lower dose of 10 mg/kg. An intermediate dose of 50 mg/kg was also chosen because of the possibility that ICH may require a higher therapeutic dose than a systemic disease.
In this study, we found that systemic treatment of 50 or 100 mg/kg DFX significantly reduced ICH-induced perihematomal brain edema at 3 days after ICH, and reduced ipsilateral ventricle enlargement and caudate atrophy 2 months after ICH compared to the vehicle-treated rats with ICH. And it also improved behavioral outcomes after ICH. On the other hand, the ICH rats treated with 10 mg/kg DFX showed significant brain edema reduction and attenuation of ipsilateral ventricle enlargement compared to vehicle-treated ICH rats, however, caudate atrophy was not significantly reduced, and residual neurological deficit was present at 56 days after ICH in the corner turn test. These results indicate that a dose higher than 10 mg/kg is the optimal dose of DFX in this model.
DFX, an iron chelator, is a drug for the treatment of acute iron intoxication and of chronic iron overload due to transfusion-dependent anemias. DFX can rapidly penetrate the blood-brain barrier and accumulate in the brain tissue at a significant concentration after systemic administration7, 8. DFX chelates iron by forming a stable complex that prevents the iron entering into further chemical reactions. However, DFX may cause hypersensitivity reactions, systemic allergic reactions, cardiovascular, hematologic and neurological adverse reactions. Serious adverse reactions include significant hypotension and marked body weight loss. In the present study, all three of the DFX doses used did not cause hypotension and or significantly affect body weight in the aged ICH rats.
In summary, systemic administration of DFX reduced ICH-induced brain edema, neurological deficits and brain atrophy without causing severe side effects in aged rats. These results suggest that the protection offered by DFX against ICH-induced brain injury occurs irrespective of age and that iron chelation with DFX could be a new therapy for ICH.
Acknowledgment
This study was supported by grants NS-017760, NS-039866, NS-047245, and NS-052510 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurgery Clinics of North America. 2002;13:371–383. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 3.Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: The role of iron. J Neurosurg. 2006;104:305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- 4.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Eng J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 7.Palmer C, Roberts RL, Bero C. Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke. 1994;25:1039–1045. doi: 10.1161/01.str.25.5.1039. [DOI] [PubMed] [Google Scholar]
- 8.Keberle H. The biochemistry of desferrioxamine and its relation to iron metabolism. Ann NY Acad Sci. 1964;119:758–768. doi: 10.1111/j.1749-6632.1965.tb54077.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang F, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: Role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res. 2005;1039:30–36. doi: 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: Effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. 2007;38:2861–2863. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- 13.Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- 14.Howard G, Toole JF, Frye-Pierson J, Hinshelwood LC. Factors influencing the survival of 451 transient ischemic attack patients. Stroke. 1987;18:552–557. doi: 10.1161/01.str.18.3.552. [DOI] [PubMed] [Google Scholar]
- 15.Gong Y, Hua Y, KeKep RF, Hoff JT, Xi G. Intracerebral hemorrhage: Effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 16.Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, Garcia NM, Morgenstern LB. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68:1651–1657. doi: 10.1212/01.wnl.0000261906.93238.72. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y, Tian H, Xi G, Keep RF, Hoff JT, Hua Y. Systemic zinc protoporphyrin administration reduces intracerebral hemorrhage-induced brain injury. Acta Neurochirurgica. 2006;96 (Suppl):232–236. doi: 10.1007/3-211-30714-1_50. [DOI] [PubMed] [Google Scholar]
- 18.Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Xi G, Park JW, Hua Y, Hoff JT, Keep RF. Holo-transferrin and thrombin can interact to cause brain damage. Stroke. 2005;36:348–352. doi: 10.1161/01.STR.0000153044.60858.1b. [DOI] [PubMed] [Google Scholar]
- 20.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. New England Journal of Medicine. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 21.Clasen RA, Huckman MS, Von Roenn KA, Pandolfi S, Laing I, Clasen JR. Time course of cerebral swelling in stroke: A correlative autopsy and ct study. Advances in Neurology. 1980;28:395–412. [PubMed] [Google Scholar]
- 22.Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–1173. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]
- 23.Enzmann DR, Britt RH, Lyons BE, Buxton JL, Wilson DA. Natural history of experimental intracerebral hemorrhage: Sonography, computed tomography and neuropathology. Ajnr: Am J Neuroradiol. 1981;2:517–526. [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE, Broderick JP, Brott TG. Lobar intracerebral hemorrhage model in pigs: Rapid edema development in perihematomal white matter. Stroke. 1996;27:490–497. doi: 10.1161/01.str.27.3.490. [DOI] [PubMed] [Google Scholar]
- 25.Tomita H, Ito U, Ohno K, Hirakawa K. Chronological changes in brain edema induced by experimental intracerebral hematoma in cats. Acta Neurochir - Suppl. 1994;60:558–560. doi: 10.1007/978-3-7091-9334-1_154. [DOI] [PubMed] [Google Scholar]
- 26.Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: Relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- 27.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? Journal of Neurochemistry. 2003;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 28.Skriver EB, Olsen TS. Tissue damage at computed tomography following resolution of intracerebral hematomas. Acta Radiologica: Diagnosis. 1986;27:495–500. doi: 10.1177/028418518602700502. [DOI] [PubMed] [Google Scholar]
- 29.Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J. Cell death in experimental intracerebral hemorrhage: The "Black hole" Model of hemorrhagic damage. Annals of Neurology. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- 30.Xi G, Fewel ME, Hua Y, Thompson BG, Hoff J, Keep R. Intracerebral hemorrhage: Pathophysiology and therapy. Neurocritical Care. 2004;1:5–18. doi: 10.1385/ncc:1:1:5. [DOI] [PubMed] [Google Scholar]
- 31.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]