Scheme 5.
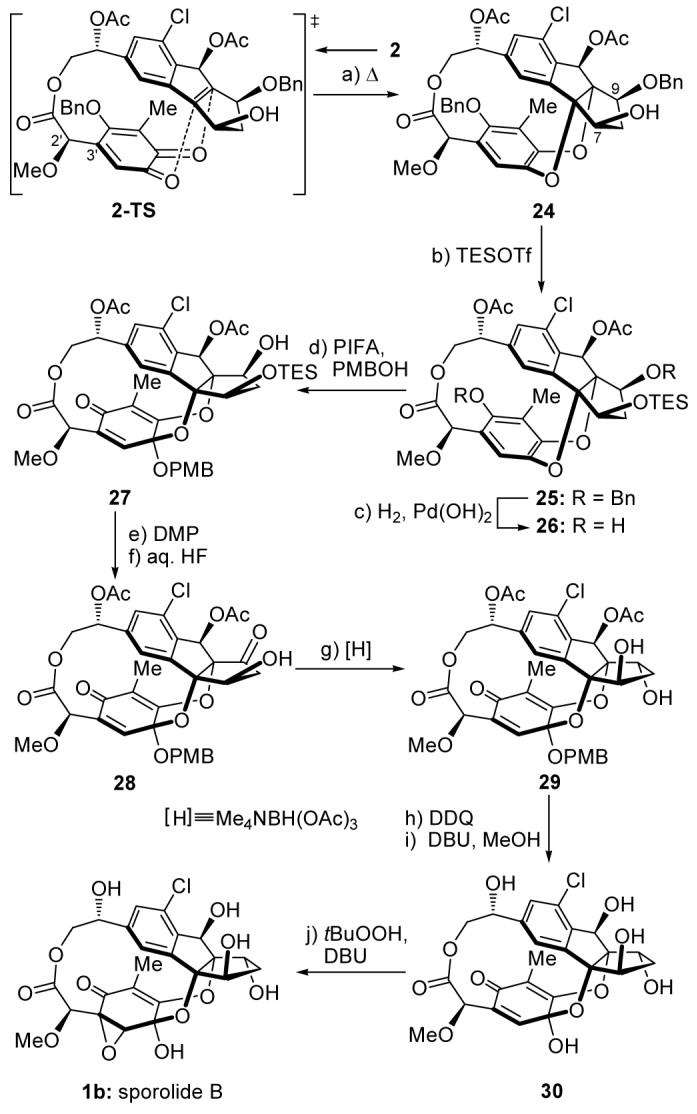
Completion of the total synthesis of sporolide B (1b). Reagents and conditions: a) toluene, 110 °C, 1.5 h, 40 % (based on 50 % recovered starting material); b) TESOTf (1.5 equiv), Et3N (2.0 equiv), CH2Cl2, 0 °C, 30 min, 95 %; c) H2 (balloon pressure), Pd(OH)2 (10 % on carbon, 2.0 equiv), EtOAc, 25 °C, 4 h, 92 %; d) PIFA (1.5 equiv), PMBOH (10 equiv), K2CO3 (5.0 equiv), MeCN, 0 °C, 30 min, 75 %; e) DMP (2.0 equiv), CH2Cl2, 25 °C, 1 h, 90 %; f) HF (48 % aqueous solution, excess), MeCN, 25 °C, 2 h, 85 %; g) Me4NBH(OAc)3 (10 equiv), MeCN/AcOH (10:1), 25 °C, 2 h, 85 %; h) DDQ (5.0 equiv), CH2Cl2/H2O (10:1), 25 °C, 5 h, 70 %; i) DBU (10 equiv), CH2Cl2/MeOH (3:1), 40 °C, 4 h, 78 %; j) tBuOOH (10 equiv), DBU (5.0 equiv), CH2Cl2, 40 °C, 3 h, 63 %. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DDQ = 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, PIFA = phenyliodine(III) bis(trifluoroacetate), PMB = 4-methoxybenzyl, Tf = trifluoromethanesulfonyl.
