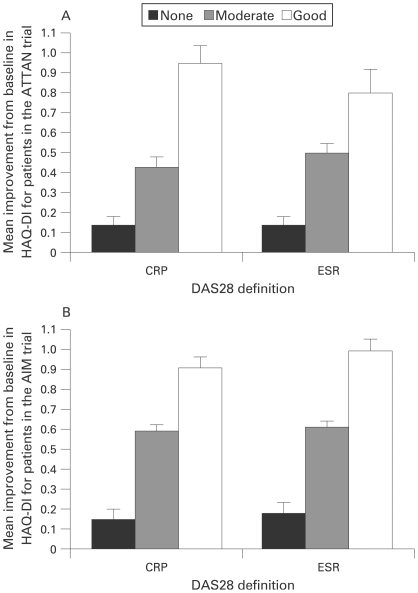
Figure 2.
Improvements in physical function across European League Against Rheumatism (EULAR) states based on 28-joint Disease Activity Score (DAS28) C-reactive protein (CRP) and DAS28 erythrocyte sedimentation rate (ESR) for patients in the ATTAIN (Abatacept Trial in Treatment of Anti-TNF INadequate responders) and AIM (Abatacept in Inadequate responders to Methotrexate) trials (both treatment groups). Mean improvement from baseline in Health Assessment Questionnaire Disability Index (HAQ-DI), for combined abatacept and patients treated with placebo who were EULAR good, moderate or non-responders based on DAS28 (CRP) or DAS28 (ESR). A. Mean improvement from baseline to 6 months for patients in the ATTAIN trial. B. Mean improvement from baseline to 6 months for patients in the AIM trial. Error bars represent the standard error of the mean (SEM).