Abstract
Fetal alcohol syndrome is a leading cause of mental retardation, but mechanisms of alcohol-associated brain damage remain elusive. Chronic alcohol exposure attenuates fetal and neonatal hypoxic cerebral vasodilation in sheep. We therefore hypothesized that alcohol could alter development of cerebrovascular responses to adenosine, a putative mediator of hypoxic cerebral vasodilation. The objective of this study was to examine the effect of earlier fetal alcohol exposure on later reactivity to adenosine in fetal sheep cerebral arterioles. Penetrating intracerebral arterioles were harvested from the brains of third trimester fetal sheep previously exposed in the second trimester to maternal alcohol “binges” (1.5g/kg IV over 90 minutes, 5 days/week for 4 weeks) or same-volume saline infusions. Arterioles were cannulated with a micropipette system and luminally pressurized. Fetal alcohol exposure did not affect spontaneous myogenic tone, but enhanced the dilator response of penetrating arterioles to extraluminal acidosis (pH 6.8). Alcohol exposure also resulted in an increase in maximal vessel response to CGS-21680, an adenosine A2A receptor agonist, but did not alter the concentration-dependent response curves to adenosine. Our results suggest that earlier alcohol exposure does not impair the subsequent responsiveness of fetal cerebral arterioles to vasodilator agents. Thus, alteration in cerebral vascular response to hypoxia in fetal sheep may not be attributed to changes in vascular reactivity to adenosine.
Keywords: Fetal Alcohol Syndrome, cerebral blood flow, sheep, adenosine, vasculature
1. Introduction
Alcohol is detrimental to the developing brain, and remains the leading cause of mental retardation in developed countries (Abel, 1997; Warren et al., 1988). The mechanisms by which in utero alcohol exposure adversely affects fetal brain development and function are largely unknown. Most studies of fetal alcohol syndrome have focused on alcohol’s direct or indirect toxic effects on developing nerves and brain structures. However, chronic alcohol exposure has well known adverse effects on brain blood vessels (Daft et al., 1986; Turcotte et al., 2002; Vorbrodt et al., 2001). Because of the overall importance of cerebrovascular regulation in brain function and development (Iadecola, 2004; Pearce, 2006), the deleterious vascular effects of alcohol may contribute to the wide range of neuropathology reported in individuals exposed to alcohol prenatally.
Few studies have examined the cerebrovascular effects of fetal alcohol exposure. We have developed a sheep model of binge fetal alcohol exposure and previously reported that early fetal alcohol exposure is associated with later attenuation of cerebral blood flow (CBF) responses to hypoxia in both fetuses (Mayock et al., 2007) and newborns (Gleason et al., 1997). Cerebrovascular hypoxic vasodilation is believed to be mediated, in part, by adenosine (Blood et al., 2003; Morii et al., 1987; Pearce, 2006). Its actions are mediated by specific cell surface receptors coupled to G-proteins, including A1, A2A, A2B and A3 subtypes (Fredholm et al., 2001). Brain extracellular adenosine levels are normally low, with a measured concentration of approximately 50 nM (Latini et al., 2001). With increased tissue metabolic activity, hypoxia or ischemia, adenosine concentrations can increase over 100 fold (Latini et al., 2001), leading to cerebral vasodilation by activating adenosine receptors that are primarily of the A2A subtype (Ngai et al., 2001).
We therefore hypothesized that alcohol exposure may alter adenosine reactivity in fetal cerebral vessels. In the present study, we evaluated the reactivity of fetal sheep cerebral arterioles to adenosine and to CGS-21680, an adenosine A2A receptor agonist. Concentration-dependent responses of arterioles isolated from third trimester fetal sheep which had been exposed to alcohol in the second trimester were determined and compared with responses from fetal sheep exposed to saline infusion.
2. Results
Maternal Infusions
Maternal blood alcohol levels, both pre- and post- infusion, were similar on days 2 and 5 of infusion and over the four weeks of the maternal infusion protocol and, therefore, were averaged. Maternal blood alcohol levels averaged 201 ± 6 mg/dL after the 1.5 hour alcohol infusion. (Table I) Maternal blood glucose levels decreased significantly after alcohol infusion. No changes in maternal blood glucose levels were noted in saline-control animals. Maternal blood lactate levels decreased in the saline-control ewes, (Table I) whereas they did not change significantly after alcohol infusion.
Table I.
Maternal Metabolic Measurements During Saline or Alcohol Infusion
| Saline | Alcohol | |||
|---|---|---|---|---|
| Preinfusion | Postinfusion | Preinfusion | Postinfusion | |
| Glucose (mg/dL) | 36.7± 1.2 | 38.3 ± 0.8 | 41.2 ± 1.6 | 29.9 ± 1.7 ** |
| Lactate (mg/dL) | 11.6 ± 1.8 | 7.2 ± 0.6* | 11.4 ± 0.7 | 15.1 ± 1.7 |
| Alcohol(mg/dL) | 0 ± 0 | 0 ± 0 | 2 ± 1 | 201 ± 6 *** |
Maternal blood concentrations before and following infusion between 60 and 90 days gestation. Values are averages from the four weeks of infusion and presented as mean ± SEM.
p = 0.049,
p = 0.0014,
p < 0.0001 compared to preinfusion values.
Fetal Cerebral Arteriole Studies
We studied 10 penetrating cerebral arterioles from saline-control fetuses and 9 arterioles from alcohol-exposed fetuses. When the circulating bath temperature was raised to normal fetal temperature (40°C) and vessels were pressurized to 40 mmHg, viable vessels contracted spontaneously to ~65% of passive (maximally dilated) diameter. Arterioles developed similar myogenic resting tone in both groups (Table II). However, vessel response to acidotic buffer (pH 6.8) was greater in the fetuses exposed to alcohol vs. saline (Table II). Arterioles from both groups exhibited robust responses to adenosine and to CGS-21680. However, the maximum vasodilator response to CGS-21680 was significantly greater in the alcohol exposed group (Table II and Figure 1).
Table II.
Fetal Cerebral Arteriole Diameter and Reactivity
| Saline-Exposed | Alcohol-Exposed | |
|---|---|---|
| N | 10 | 9 |
| Passive Diameter (μm) | 142 ± 16 | 157 ± 10 |
| Dilation to pH 6.8 a | 26 ± 3 % | 35 ± 3 % * |
| Resting Tone (% passive diameter) | 65 ± 2 % | 67 ± 2 % |
| Maximal Adenosine Dilation a | 52 ± 7% | 51 ± 5% |
| Adenosine LogEC50 | −6.35 ± 0.19 | −6.48 ± 0.14 |
| Maximal CGS-21680 Dilation a | 32 ± 5% | 53 ± 8% * |
| CGS-21680 LogEC50 | −7.71 ± 0.17 | −7.56 ± 0.74 |
Values presented as mean ± SEM.
Dilation responses expressed as % of resting diameter (diameter measured after the development of steady spontaneous tone).
p < 0.05 versus saline-exposed vessels.
Figure 1. Cerebral Arteriolar Dilatory Response to Adenosine Agonists.
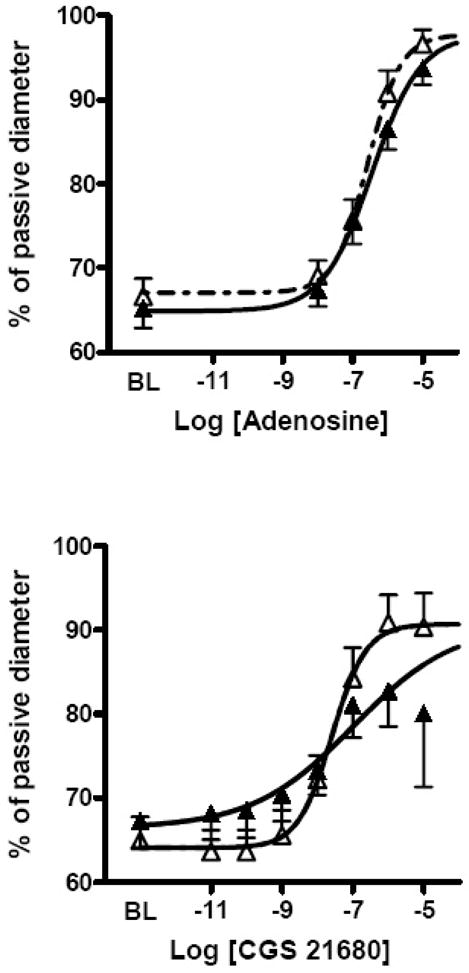
Top: Vessel response to adenosine.
Bottom: Vessel response to CGS-21680.
Saline-control fetuses: Solid curve with closed symbols. Alcohol-exposed fetuses: Dashed curve with open symbols. Standard error flags are shown. Resting tone is similar in both groups of vessels. Values are means ± SEM.
3. Discussion
This paper provides the first report on the vasomotor properties of penetrating cerebral arterioles in the fetal sheep and specifically reports on the effects of prior alcohol exposure. We found that exposure to maternal alcohol binges during midgestation does not alter the ability of late gestation fetal arterioles to develop myogenic tone in vitro, nor the reactivity of these arterioles to adenosine. However, alcohol exposed arterioles reacted differently to a selective A2A adenosine receptor agonist and to H+ compared to control vessels.
We chose a 2nd trimester binge alcohol model for several reasons. First, binge drinking is more clinically relevant than continuous alcohol exposure (Caetano et al., 2006). For reference, the amount of alcohol needed to bring human alcohol blood level to 200 mg/dL – as in our sheep – is 7 ounces of hard liquor or 7 beers consumed over a 90 minute period. The legal limit in the United States for blood alcohol content while driving is 80 mg/dL. Second, the 2nd trimester may be a particularly vulnerable time for brain injury since alcohol-induced neuronal loss in rats is reportedly the greatest with prenatal alcohol exposure during the 2nd trimester (Miller et al., 1990). In sheep, the brain growth spurt also occurs in the second trimester (Dobbing et al., 1979). Taken together, the most vulnerable period with respect to brain growth in the developing fetal sheep brain is thus likely to be in the 2nd trimester.
The penetrating cerebral arterioles are considered important contributors to cerebral vascular resistance in vivo (Dacey et al., 1982; Faraci et al., 1990). We used the pressure myograph technique, which is considered more physiologic than vessel strips or rings (Dunn et al., 1994; Mulvany et al., 1990), and allows us to investigate the vasomotor responses of cerebral arterioles in isolation from the possible confounding influences of metabolic and humoral factors. This methodology has been utilized extensively to study the role of various vasoactive agents and metabolites on cerebral arteriolar reactivity in rodents (Coyne et al., 2002; Dacey et al., 1982; Ngai et al., 2001). Although no comparable data is available in fetal sheep penetrating arterioles, cerebral arterioles in this study developed levels of myogenic tone similar to isolated adult vessels from rodents (Coyne et al., 2002; Dacey et al., 1982; Ngai et al., 2001). Additionally, the response of fetal sheep intracerebral arterioles to extraluminal pH decrease (Table II) is comparable to the pH reactivity of pial arterioles in vivo (Lindauer U et al., 2003) and rat arterioles in vitro (Ngai et al., 2001). Marked responsivity to extracellular pH changes, which underlies cerebral CO2 reactivity, is a characteristic of cerebral vessels (Lindauer et al., 2003). The mechanism by which extraluminal H+ dilates cerebral vessels remains unclear, but recent evidence suggests that nitric oxide or potassium channels may be involved (Lindauer et al., 2003). In the present study, fetal alcohol exposure significantly enhanced the dilation response of sheep cerebral arterioles to a decrease in extraluminal pH. Further studies are needed to determine how fetal alcohol exposure may alter cerebral vessel sensitivity to pH changes.
In this study, adenosine dilated fetal sheep penetrating arterioles in a concentration-dependent manner, with –log EC50 values that are similar to those reported in rat intracerebral arterioles (Ngai et al., 2001). In control arterioles, adenosine, which is capable of activating A2A and A2B adenosine receptors (Fredholm et al., 2001), induced a greater maximal dilation than the A2A receptor specific agonist, CGS-21680. The greater efficacy of adenosine (vs. CGS-21680) has been observed in rat cerebral arterioles (Ngai et al., 2001), and suggests that A2B receptors may participate in adenosine-induced dilation in cerebral arterioles (Ngai et al., 2001). On the other hand, alcohol exposure appeared to have selectively enhanced the efficacy of CGS-21680 in eliciting dilation of fetal cerebral arterioles without affecting the maximal response to adenosine. The differential effect of fetal alcohol on A2A receptor function is difficult to explain, but because both A2A and A2B receptors may participate in the dilation response to adenosine, one possible explanation is that the enhanced efficacy of A2A receptor activation may be offset by a down-regulation in adenosine-A2B receptor expression. Indeed, there is precedent for pathological stimuli to modulate adenosine receptor expression and selectively enhance or reduce specific receptor subtypes. For example, hypoxia has been shown to downregulate the expression of A2A receptors in human endothelial and smooth muscle cells while increasing expression of A2B receptors (Feoktistov et al., 2004). Further studies will be needed to investigate the pharmacological and molecular mechanisms involved.
We previously demonstrated that fetal alcohol exposure attenuates the vascular response of the cerebral vasculature to hypoxia (Gleason et al., 1997; Mayock et al., 2007). Because cerebrovascular hypoxic vasodilation is mediated, in part, by adenosine (Blood et al., 2003; Morii et al., 1987; Pearce, 2006), we hypothesized that alcohol exposure might affect vasodilatory responses of cerebral vessels to adenosine. Instead, the present findings reveal that alcohol exposure did not alter the response of fetal sheep cerebral arterioles to endogenous vasoactive mediators, including adenosine. It is possible, however, that chronic alcohol exposure may alter other putative vasodilator mechanisms, such as nitric oxide (Blood et al., 2003; Endres et al., 2004), leading to alteration of cerebral blood flow responses to hypoxia.
In summary, we have demonstrated that fetal alcohol exposure during the second trimester does not alter cerebral arteriolar reactivity to adenosine later in gestation. However, alcohol exposure does alter vessel responses to acidosis and to a selective A2A adenosine receptor agonist. Further studies are needed to clarify the role of various adenosine receptor subtypes in this response.
4. Experimental Procedure
All experimental protocols were approved by the University of Washington and University of Idaho Animal Care and Use Committees. Mixed breed fetal sheep were obtained from time dated pregnancies. Full term gestation is 150 days.
Maternal Infusion Protocol
Pregnant sheep were randomized to receive daily (Monday through Friday) intravenous infusions of either alcohol (n = 8) or saline (n = 9) from 60 to 90 days gestation. Ewes were bred, housed and received IV alcohol or saline infusions at the animal research facilities of the University of Idaho, Department of Animal and Veterinary Science, Center for Reproductive Biology in Moscow, Idaho. A jugular venous catheter was placed percutaneously under xylazine (0.1 mg/kg IM) and local lidocaine anesthesia on day 60 of gestation for the duration of the infusion protocol. Catheter patency was maintained by heparin flush (100 Units/mL). Alcohol (1.5 grams pure ethanol/kg ewe weight, 33% v/v solution) or the equivalent volume of normal saline was infused over 1.5 hours. Once the daily infusion protocol was complete, the jugular catheter was removed.
Maternal blood levels of glucose, lactate and alcohol were measured before and after infusion on the second and last days of each infusion week. Samples were obtained during all four weeks of infusion. Blood samples were immediately deproteinized, centrifuged, and the supernatant decanted, frozen, and shipped on dry ice to the University of Washington, where they were stored at −20°C until assayed. Blood glucose, lactate, and alcohol concentrations were measured enzymatically by glucose oxidase, lactate dehydrogenase, and alcohol dehydrogenase oxidation utilizing standard assay kits (Sigma Diagnostics, St. Louis, MO or Diagnostic Chemical Limited, Oxford, CN).
Fetal Vessel Preparation
Fetal penetrating cerebral arterioles were obtained for study on days 125–128 gestation. The ewe was deeply anesthetized with pentobarbital after a 16-gauge venous catheter was placed percutaneously in a maternal jugular vein for fluid administration. The trachea was intubated and the ewe was mechanically ventilated. The uterus was exposed through a midline incision. Cesarean delivery of the fetus was accomplished and an overdose of pentobarbital was injected into the umbilical vein. The fetus then underwent immediate decapitation and removal of the brain. A wedge of cerebral cortex ~4 mm thick at the base of the brain extending from the Circle of Willis to the lateral cerebral artery and containing the middle cerebral artery was dissected bilaterally. The pia mater and attached penetrating intracerebral arterioles were separated from the parenchyma. Intracerebral arterioles (1–2 mm in length and 80–250 μm in diameter) were excised and placed in a temperature-controlled vessel chamber on the stage of an inverted microscope. The vessel was cannulated at both ends with concentric micropipettes consisting of a perfusion pipette within a holding pipette (Ngai et al., 2001; Ngai et al., 1993). After cannulation, the vessel was pressurized to 40 mmHg and perfused intraluminally at a rate of 2 μL/min with buffered salt solution (pH 7.4) containing 1% bovine serum albumin. The vessel bath contained MOPS-buffered salt solution (pH 7.4) replaced with fresh buffer at 1 mL/min. The composition of the 3-(N-morpholino)-propanesulfonic acid (MOPS) buffered saline solution was as follows (in mM): 144.0 NaCl, 3.0 KCl, 2.5 CaCl2, 1.5 MgSO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 2.0 MOPS, and 1.2 NaH2PO4, pH 7.4. Adenosine, CGS-21680, and MOPS were obtained from Sigma (St. Louis, MO).
After measurement of passive internal vessel diameter with a video micrometer, the bath temperature was raised to 40°C (normal fetal sheep temperature). Viable vessels gradually develop spontaneous myogenic tone and constrict to a stable baseline diameter of ≤68% of passive diameter. Vessel reactivity was assessed by replacing the bath fluid with acidic (pH 6.8) buffer. Viable vessels dilated to ≥115% of baseline diameter. Vessels not meeting viability criteria were discarded.
We analyzed the responses of vessels to adenosine and to the A2A receptor-specific agonist CGS-21680. Adenosine (1mM) and CGS-21680 (0.1mM) were dissolved directly into the MOPS-buffered salt solution at pH 7.4. Serial subsequent dilutions were made with MOPS. Adenosine test range was 10−8 to10−4 M/L and CGS-21680 test range was 10−11 to 10−5 M/L. Concentration response curves were constructed by changing the bath solution in 10-fold concentration increments by means of a perfusion pump at 1 mL/min. Vessel diameter was recorded at 6 minutes after each solution change. After the response to the highest agonist concentration (0.1 mM for adenosine and 0.01 mM for CGS-21680) were determined, the bath conditions were restored to baseline. The experiment was discontinued if vessel diameter did not recover to ± 10% of control within 20 minutes after washout.
Statistical Evaluation
All data are expressed as means ± SEM. Dilator responses are expressed as a percentage of resting tone (diameter measured after the development of steady spontaneous tone). We utilized GraphPad Prism™ (GraphPad Software, San Diego, CA) to enter and analyze data. This program analyzes sigmoidal dose-response (variable slope) best-fit values using a 4-parameter (top, bottom, logEC50 and slope) logistic (‘Hill’) equation and the F-test. Using two-tailed Student’s t-test, we compared parameters for vessels from alcohol-exposed versus saline-control fetal sheep brains. A p value <0.05 was considered significant. Vessels were excluded when the logEC50 95% confidence interval was not defined or was greater than 2 standard deviations away from the mean logEC50 confidence interval.
Acknowledgments
This study was supported by the National Institutes of Health – National Institute of Alcohol Abuse and Alcoholism Grant RO1-AA12403. We wish to express our thanks for the expert technical assistance of Richard Tuck, Erica Gordon, Yuh-Chi Niou, and Than-Poh Chong.
Abbreviations
- CGS-21680
2-p-(2-carboxyethyl)-phenethylamino-5′-N-ethylcarboxyamido-adenosine
- MOPS
3-(N-morpholino)-propanesulfonic acid
- LogEC50
log concentration of agonist required to elicit 50% of maximum response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Was the fetal alcohol syndrome recognized in the ancient Near East? Alcohol Alcohol. 1997;32:3–7. doi: 10.1093/oxfordjournals.alcalc.a008231. [DOI] [PubMed] [Google Scholar]
- Blood AB, Hunter CJ, Power GG. Adenosine mediates decreased cerebral metabolic rate and increased cerebral blood flow during acute moderate hypoxia in the near-term fetal sheep. J Physiol. 2003;553:935–945. doi: 10.1113/jphysiol.2003.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res. 2006;30:1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Coyne EF, Ngai AC, Meno JR, Winn HR. Methods for isolation and characterization of intracerebral arterioles in the C57/BL6 wild-type mouse. J Neurosci Methods. 2002;120:145–153. doi: 10.1016/s0165-0270(02)00197-8. [DOI] [PubMed] [Google Scholar]
- Dacey RG, Jr, Duling BR. A study of rat intracerebral arterioles: methods, morphology, and reactivity. Am J Physiol Heart Circ Physiol. 1982;243:H599–H606. doi: 10.1152/ajpheart.1982.243.4.H598. [DOI] [PubMed] [Google Scholar]
- Daft PA, Johnston MC, Sulik KK. Abnormal heart and great vessel development following acute ethanol exposure in mice. Teratology. 1986;33:93–104. doi: 10.1002/tera.1420330112. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Wellman GC, Bevan JA. Enhanced resistance artery sensitivity to agonists under isobaric compared with isometric conditions. Am J Physiol. 1994;266:H147–H155. doi: 10.1152/ajpheart.1994.266.1.H147. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke prevention. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension. 2004;44:649–654. doi: 10.1161/01.HYP.0000144800.21037.a5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gleason CA, Iida H, Hotchkiss KJ, Northington FJ, Traystman RJ. Newborn cerebrovascular responses after first trimester maternal ethanol exposure in sheep. Pediatr Res. 1997;42:39–45. doi: 10.1203/00006450-199707000-00007. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Vogt J, Schuh-Hofer S, Dreier JP, Dirnagl U. Cerebrovascular vasodilation to extraluminal acidosis occurs via combined activation of ATP-sensitive and Ca2+-activated potassium channels. J Cereb Blood Flow Metab. 2003;23:1227–1238. doi: 10.1097/01.WCB.0000088764.02615.B7. [DOI] [PubMed] [Google Scholar]
- Mayock DE, Ngai AC, Mondares RL, Gleason CA. Binge alcohol exposure in the second trimester attenuates fetal cerebral blood flow response to hypoxia. J Appl Physiol. 2007;102:972–977. doi: 10.1152/japplphysiol.00956.2006. [DOI] [PubMed] [Google Scholar]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol. 1990;239:92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Morii S, Ngai AC, Ko KR, Winn HR. Role of adenosine in regulation of cerebral blood flow: effects of theophylline during normoxia and hypoxia. Am J Physiol. 1987;253:H165–H175. doi: 10.1152/ajpheart.1987.253.1.H165. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev. 1990;70:921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Coyne EF, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Winn HR. Effects of adenosine and its analogues on isolated intracerebral arterioles. Circ Res. 1993;73:448–457. doi: 10.1161/01.res.73.3.448. [DOI] [PubMed] [Google Scholar]
- Pearce WJ. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol. 2006;100:731–738. doi: 10.1152/japplphysiol.00990.2005. [DOI] [PubMed] [Google Scholar]
- Turcotte LA, Aberle NS, Norby FL, Wang GJ, Ren J. Influence of prenatal ethanol exposure on vascular contractile response in rat thoracic aorta. Alcohol. 2002;26:75–81. doi: 10.1016/s0741-8329(01)00198-7. [DOI] [PubMed] [Google Scholar]
- Vorbrodt AW, Dobrogowska DH, Kozlowski P, Tarnawski M, Dumas R, Rabe A. Effect of a single embryonic exposure to alcohol on glucose transporter (GLUT-1) distribution in brain vessels of aged mouse. J Neurocytol. 2001;30:167–174. doi: 10.1023/a:1011995308851. [DOI] [PubMed] [Google Scholar]
- Warren KR, Bast RJ. Alcohol-related birth defects: an update. Public Health Rep. 1988;103:638–642. [PMC free article] [PubMed] [Google Scholar]


