Abstract
The introduction of antigen into the anterior chamber (AC) of the eye induces the production of antigen-specific splenic CD8+ regulatory T cells (AC-SPL cells) that suppress a delayed-type hypersensitivity (DTH) reaction in immunized mice. Because the generation of these regulatory T cells is also induced by exposure to transforming growth factor (TGF)-β and antigen or F4/80+ cells exposed to TGF-β and antigen in vitro, we investigated (i) whether these cells are produced in dominant negative receptor for transforming growth factor β receptor type II (dnTGFβRII) or Cbl-b−/− mice whose T cells are resistant to TGF-β, (ii) whether DTH is suppressed by wild type (WT) CD8+ AC-SPL cells in Cbl-b−/− and dnTGFβRII mice and (iii) the effect of antibodies to TGF-β on the suppression of DTH by CD8+ AC-SPL cells. DnTGFβRII immunized and Cbl-b−/− mice produced splenic CD8+ regulatory cells after the intracameral injection of antigen and immunization. The suppression of a DTH reaction by CD8+ AC-SPL cells in WT mice was blocked by the local inclusion of antibodies to TGF-β when WT splenic CD8+ AC-SPL cells were injected into the DTH reaction site. Moreover, the DTH reaction in immunized dnTGFβRII and Cbl-b−/− mice was not suppressed by the transfer of WT CD8+ AC-SPL cells to the site challenged with antigen. In aggregate, these observations suggest that T cell sensitivity to TGF-β is not an obligate requirement for the in vivo induction of CD8+ AC-SPL T cells but the suppression of an in vivo DTH reaction by CD8+ AC-SPL cells is dependent on TGF-β.
Keywords: regulatory T cell, TGF-β, tolerance/suppression
Introduction
The antigen-specific suppression of T cells to self and non-self antigens in Th1 and Th2 immune responses is effected by CD8+ regulatory T cells that are distinct from CD8+ cytotoxic T cells (1–6). In addition to induction, CD8+ regulatory T cells, unlike CD4+, CD25+, Foxp3+ regulatory T cells, suppress immunized T cells (7). Moreover, the immunosuppressive activity of CD8+ regulatory T cells is detected after an immune response to the antigen (1, 4), suggesting that antigen-specific CD8+ regulatory T cells are induced and/or amplified during an immune response but the requirements and/or mechanisms of the activation of these cells in vivo are not clear.
CD8+ regulatory T cells are induced by the injection of antigen into the anterior chamber (AC) of an eye [AC-SPL cells (6, 8–12)] or in vitro by the ligation of 4-1BB (13, 14), transforming growth factor (TGF)-β (15) or TGF-β, antigen-pulsed F4/80+ cells (16). The immunosuppressive properties of TGF-β (17) suggest that this cytokine could be a suppressive mechanism used in vivo by CD8+ regulatory T cells. Accordingly, TGF-β potentially could have an afferent and/or efferent role in the immunosuppression mediated by CD8+ regulatory T cells.
In the current studies, we investigated requirements for T cell sensitivity to TGF-β for the generation in vivo of splenic CD8+ regulatory T cells induced by the injection of antigen into the AC by using two genetically engineered strains of mice, dominant negative receptor for transforming growth factor β receptor type II (dnTGFβRII) or Cbl-b−/−, resistant to TGF-β. In the second strain, the E3 ubiquitin ligase Cbl-b has been specifically deleted [Cbl-b−/− mice (18, 19)]. Cbl-b, a member of the Cbl/Sli family of molecular adaptors and a ubiquitin ligase, has been shown to negatively regulate CD28-dependent T cell activation. Recently, we reported that Cbl-b−/− T cells, though not constitutively activated, are resistant to the inhibitory effects of TGF-β (20, 21). Cbl-b−/− T cells display increased proliferation after TCR stimulation and produce increased amounts of IL-2, but not IFN-β or tumor necrosis factor-α. Cbl-b−/− mice have T cells that are independent of CD28 co-stimulation, in that they do not require CD28 engagement for IL-2 production and proliferation (18, 19). Moreover, Cbl-b−/− mice develop spontaneous autoimmunity characterized by auto-antibody production, infiltration of activated T and B lymphocytes into multiple organs, and parenchymal damage and are highly susceptible to experimental autoimmune encephalomyelitis (18, 19). In the second TGF-β-resistant strain, mice transgenically express a dnTGFβRII in CD4+ and CD8+ T cells (22). This dominant negative TGF-β receptor prevents TGF-β from signaling normally in T cells and T cells from dnTGFβRII mice respond well to antigen but do not respond to TGF-β. A significant percentage of the T cells from dnTGFβRII mice show evidence of prior activation in terms of CD44 expression (22).
We show that (i) dnTGFβRII and Cbl-b−/− mice that received an intracameral injection of antigen produce CD8+ AC-SPL cells that suppress a DTH reaction when transferred to wild-type (WT) recipients; (ii) The DTH reaction cannot be suppressed in immunized dnTGFβRII and Cbl-b−/− recipients by transferred WT CD8+ AC-SPL cells. (iii) Antibodies to TGF-β inhibit the local in vivo suppression of a DTH reaction by CD8+ regulatory spleen cells induced by the injection of antigen into the AC. These results suggest that sensitivity to TGF-β by T cells is not obligate for the in vivo induction of CD8+ AC-SPL cells induced via the AC but TGF-β is likely a suppressive mechanism utilized by CD8+ AC-SPL cells.
Methods
Mice
Female or male C57BL/6 mice 8–10 weeks old were purchased from Charles River/NCI Laboratories (Wilmington, MA, USA). Cbl-b-deficient mice (18, 19) on a C57BL/6 background, a gift from Dr H. Gu (National Institutes of Health, Bethesda, MD, USA) are bred in our facilities under specific, pathogen-free conditions. DnTGFβRII mice [CD4-dnTGFβRII (22)] derived from Dr Flavell's colonies at Yale University are bred in our facilities under specific, pathogen-free conditions. All mice are maintained in the Center for Laboratory Animal Care of the University of Connecticut Health Center. All work with animals was approved previously by the University of Connecticut Health Center Animal Care Committee (ACC2007-380, ACC2007-379). All animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Antigens/immunization
Trinitrophenol–BSA
2,4,6, Trinitrobenzene sulphonic acid (TNP), BSA and ovalbumin (OVA) were purchased from Sigma Chemical Co. (St Louis, MO, USA). TNP–BSA was prepared as described by Li et al. (10). Picryl chloride (PCl), 2-chloro-1,3,5-trinitorobenzene [the antigenic equivalent of TNP used to elicit contact sensitivity (CS)] was purchased as 2-chloro-5-trypthtane from Chemical Alta Ltd (Edmonton, Alberta, Canada). Mice were immunized by a single subcutaneous injection of 200 μg TNP–BSA in 50 μl CFA (Sigma–Aldrich, St Louis, MO, USA). In general, the mice were challenged to induce CS to TNP 7–9 days after the mice were immunized.
OVA
Mice received s.c. 200 μg OVA in 50 μl CFA to a flank.
Induction of DTH reaction
A CS reaction to TNP in TNP–BSA-sensitized or naive mice was induced by the epicutaneous application of 15 μl 1% PCl) in acetone:olive oil (4:1) to a footpad (10). A DTH reaction (we define the DTH reaction as the swelling induced by the application of antigen to an immunized recipient to distinguish the DTH reaction from the initiation of a Th1 response) to OVA was induced by an intra-dermal injection of 50 μg OVA in 30 μl PBS (pH 7.2). The DTH response was usually measured approximately 24–48 h after the application of PCl or injection of OVA by measuring footpad thickness with an engineer's digital micrometer (Mitatoyo, Tokyo, Japan). Before challenge, mice were anesthetized with ketamine (75 mg kg−1)/xylazine (15 mg kg−1) and the thickness of each footpad was measured. One footpad was then challenged with antigen and the other with vehicle only. Twenty-four hours later the mice were anesthetized with ketamine/xylazine and the thickness of each footpad was measured. The thickness of each footpad before the application of PCl was subtracted from that of the thickness 24 h after the application of PCl. The increase in the thickness of the footpad defined as swelling was computed as the difference in the increase in micrometers between the 24 h and 0 h measurement of the thickness of the challenged footpad minus that of the unchallenged footpad.
Injection of antigen into the AC (intracameral injection)
Mice were anesthetized with ketamine/xylazine (75 mg kg−1)/xylazine (15 mg kg−1) and, under a dissecting microscope, ∼3 μl PBS containing 4 μg TNP–BSA or 50 μg OVA was injected into the AC. A 32g needle attached to a cannula attached to a manually controlled Hamilton syringe (Stoelting Co., Wood Dale, IL, USA) was inserted into the AC. The mice recovered ∼30 min after the injection; they exhibited no distress and began eating and drinking normally. Seven days after the intracameral injection of antigen, mice were immunized with TNP–BSA as described (9, 10).
Preparation of splenic AC-induced regulatory cells (AC-SPL cells)
Fourteen days after receiving an injection of TNP–BSA into the AC and 7 days after the AC-injected mice were immunized, spleens were removed from the mice, pooled, diced and expressed through a 40-mm nylon mesh (BD Falcon, Bedford, MA, USA) with a 1-ml syringe plunger into PBS. The cells were washed 2× and suspended in PBS at 1 × 108 ml−1. The cell suspension was washed with PBS and centrifuged at 200 × g for 6–8 min. BD Pharm Lyse (BD Biosciences, San Jose, CA, USA) was used for lysing the erythrocytes according to the manufacturer's protocol. The cells were then washed twice with PBS and re-suspended in PBS. CD8+ and CD8− spleen cells were prepared by washing splenocytes two times with PBS and re-suspending the cells in BD™ IMag separation buffer (BD Biosciences, Rockville, MD, USA). The spleen cells were separated by immunomagnetic beads into CD8+ and CD8− populations with a BD™ CD8 T lymphocyte enrichment set (BD Biosciences) according to the manufacturer's protocol. Enrichment of the cells was assessed by flow cytometry and found to be >87–92% pure CD8+ T cells. The CD8− fractions showed <3% CD8+ T cells (data not shown).
Local transfer of suppression (LTS)
AC-SPL cells were assayed for the suppression of the DTH reaction by the LTS assay (10). Spleen cells were counted with a Coulter counter and unless otherwise specified 10 000 or 25 000 cells in 40 μl PBS were injected subcutaneously into the footpad of immunized mice immediately following epicutaneous challenge with PCl. Swelling was measured 24 h later.
The use of anti-TGF-β antibodies in the LTS was performed by admixing AC-SPL cells from donors that received an intracameral injection of antigen (1 × 106 ml−1 PBS) with monoclonal anti-TGF-β (TGF-β1,2,3) IgG1 antibody (MAB #1835, R&D Systems, Minneapolis, MN, USA) or isotype control MOPC 21 IgG (Sigma, St Louis, MO, USA) so that 40 μl contained 15 μg antibody or MOPC 21 isotype control. The cells were held at 4°C for 30 min and then injected intradermally into the footpad of sensitized recipients immediately after the footpad was challenged with antigen. Some immunized mice received an injection of anti-TGF-β antibody only after challenge.
Statistics
Statistical significance was calculated by one-way analysis of variance. P-values were determined by the Student–Neuman–Keuls test.
Results
Antigen-specific local suppression of the DTH reaction by CD8+ AC-SPL cells
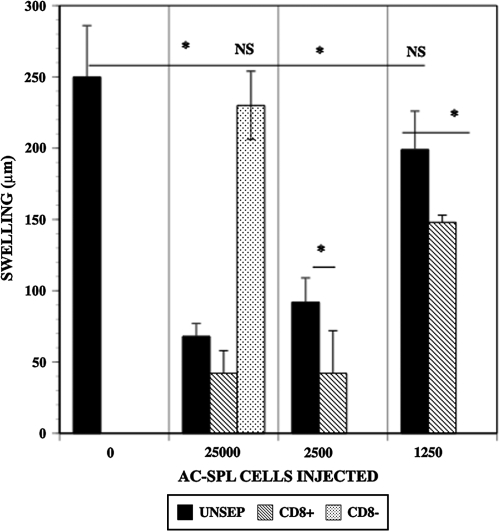
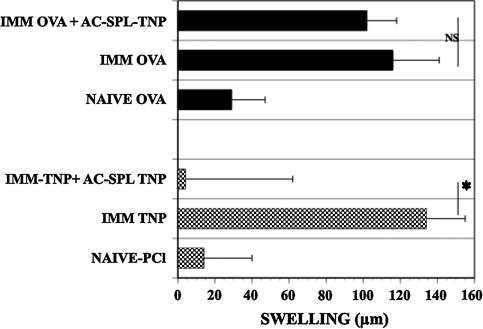
To confirm and demonstrate the phenotype of AC-SPL cells that suppress locally the DTH reaction in the LTS, graded numbers of CD8+, CD8− or unseparated AC-SPL cells from donors that received an intracameral injection of TNP–BSA were injected into the footpads of TNP–BSA-immunized mice immediately after the footpads were challenged with epicutaneous PCl. The swelling reaction in the footpads was eliminated or reduced significantly in the recipients of unseparated or CD8+ but not CD8− AC-SPL cells (Fig. 1). The enrichment for CD8+ regulatory spleen cells is shown by the suppression of PCl-induced swelling by limiting numbers (1, 250) of CD8+ AC-SPL cells while that number of unseparated AC-SPL cells did not suppress swelling significantly. CD8+ AC-SPL cells recovered from donors that received intracameral injections of TNP–BSA or OVA were assayed for the ability to suppress the DTH reaction when injected into the footpads of mice immunized to TNP–BSA or OVA after the footpad was challenged with PCl or OVA. As shown in Fig. 2, CD8+ spleen cells recovered from donors receiving intracameral TNP–BSA suppressed the swelling reaction of TNP–BSA-immunized mice challenged with PCl but not that of OVA-sensitized mice challenged with OVA. Conversely, AC-SPL cells from mice receiving intracameral OVA suppress the OVA-induced swelling in OVA-immunized mice but not PCl-induced swelling in TNP–BSA-immunized mice (data not shown). Therefore, the results in Figs 1 and 2 document further that the local, antigen-specific suppression of the DTH reaction in immunized recipients by transferred AC-SPL cells in the LTS is due to CD8+ regulatory T cells.
Fig. 1.
CD8+ AC-SPL cells suppress DTH in WT mice in the LTS. WT (C57BL/6) mice received an injection of TNP–BSA into the AC. One week later, these mice were immunized with TNP–BSA and CFA. Seven days later, the spleens were recovered from the AC-injected mice, pooled and a spleen cell suspension was prepared (AC-SPL cells). Spleen cells were separated into CD8+ and CD8− cells by immunomagnetic beads; 1.25 × 103 to 2.5 × 104 unseparated (UNSEP), CD8+ or CD8− AC-SPL cells were injected into the footpads of TNP–BSA-immunized (IMM) or naive C57BL/6 mice immediately after the footpads were challenged with epicutaneous PCl. The thickness of the footpads before and 24 h after challenge was measured to compute the swelling of the footpad. Data represents one of three experiments with similar results and is the mean ± SEM swelling of five mice per group. *P < 0.01.
Fig. 2.
Antigen-specific suppression of DTH in the LTS by CD8+ AC-SPL cells. CD8+ AC-SPL cells recovered from mice that received an intracameral injection of TNP–BSA were then immunized with TNP–BSA and injected into the footpads of TNP–BSA-immunized or OVA-immunized mice immediately after the footpad of the immunized mice was challenged with epicutaneous PCl or intra-dermal OVA, respectively. Swelling was measured 24 h after challenge. Data are the mean ± SEM for the results obtained in two experiments with eight mice per group. *P < 0.01.
AC-SPL CD8+ regulatory cells are produced by dnTGFβRII mice or Cbl-b−/− mice
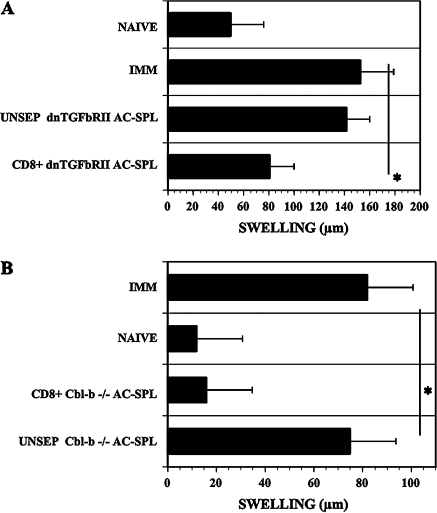
To determine whether sensitivity to TGF-β by T cells is required by the T cells in vivo to generate splenic CD8+ regulatory cells after the intracameral injection of antigen, we investigated whether CD8+ regulatory T cells are generated in dnTGFβRII and Cbl-b−/− mice after these mice received an injection of TNP–BSA into the AC. WT, dnTGFβRII or Cbl-b−/− mice received an intracameral injection of TNP–BSA. One week later, the mice were immunized with TNP–BSA and 1 week after immunizing, spleen cells were recovered from these mice and injected into the footpads of TNP–BSA-sensitized WT mice immediately after the footpads were challenged with epicutaneous PCl. Fig. 3(A and B) demonstrate that the injection of unseparated WT but not unseparated Cbl-b−/− or dnTGFβRII-derived AC-SPL cells into the footpad at a challenge site at the time of challenge resulted in a significant reduction in footpad swelling in the immunized recipient. However, swelling was suppressed in the footpads of the recipients of Cbl-b−/− or dnTGFβRII-derived CD8+ AC-SPL cells despite the fact that unseparated Cbl-b−/− or dnTGFβRII-derived CD8+ AC-SPL did not suppress the DTH reaction. Moreover, the intracameral injection of TNP–BSA into Cbl-b−/− or dnTGFβRII mice induced the production of peripheral blood mononuclear cells or thymocytes that induce splenic CD8+ AC-SPL cells (data not shown).
Fig. 3.
Intracameral injection of antigen induces CD8+ regulatory T cells in dnTGFβRII mice and Cbl-b−/− mice. (A) One week after dnTGFβRII mice received an intracameral injection of TNP–BSA, the mice were immunized with TNP–BSA and CFA. One week later, spleens were recovered, spleen cell suspensions prepared and separated into CD8+ and CD8− populations by immunomagnetic beads. IMM, immunized WT recipients received 25 000 unseparated AC-SPL cells (UNSEP) or separated AC-SPL cells. NAIVE, non-immune mice. Swelling is the mean ± SEM of data obtained in three experiments of 9–10 mice per group. *P < 0.01. (B) One week later, Cbl-b−/− mice received an intracameral injection of TNP–BSA, the mice were immunized with TNP–BSA and CFA. One week after immunization, spleens were recovered and spleen cell suspensions prepared and separated with immunomagnetic beads into CD8+ and CD8− populations. TNP–BSA-immunized recipient WT mice received an intradermal injection of 10 000 unseparated (UNSEP), CD8+ or CD8− AC-SPL cells into the challenged footpad immediately after challenge. Swelling was measured 24 h after challenge with epicutaneous PCl. Data are the mean and SEM of two experiments, six to eight mice per group. *P < 0.05.
WT CD8+ spleen cells from mice receiving intracameral antigen do not suppress DTH in TGF-β-resistant Cbl-b−/− or dnTGFβRII mice
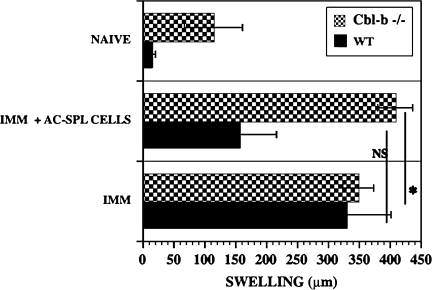
Because unseparated AC-SPL cells from TGF-βRII or Cbl-b−/− mice did not suppress the DTH reaction but CD8+ AC-SPL cells did suppress the DTH reaction when transferred to immunized recipients, we reasoned that the AC-SPL cells from TGR-βRII or Cbl-b−/− mice might contain T cells resistant to suppression effected by TGF-β. These cells could transfer a hypersensitivity reaction to the recipient mice. To investigate this hypothesis, we determined whether the DTH reaction was suppressed by AC-SPL cells in mice resistant to TGF-β. Because Cbl-b−/− mice lacking the ubiquitin ligase Cbl-b are resistant to the immunosuppressive effects of TGF-β (20, 21), spleen cells from WT donors that received intracameral TNP–BSA were injected into a footpad of TNP–BSA-immunized Cbl-b−/− or WT mice immediately after the mice were challenged with an epicutaneous application of PCl. Swelling was measured 24 h later. Immunized Cbl-b−/− and WT mice both showed robust a DTH reaction when challenged with PCl. WT spleen cells suppressed the DTH response of WT mice to naive levels but, in contrast, the DTH response of Cbl-b−/− mice receiving WT spleen cells was not suppressed (Fig. 4).
Fig. 4.
DTH in Cbl-b−/− mice is resistant to suppression by WT-AC-SPL cells. Twenty-five thousand AC-SPL cells from WT (C57BL/6), Cbl-b +/+ mice that received intracameral TNP–BSA were injected into footpads intradermally immediately after the footpads of TNP–BSA-immunized Cbl-b−/− and WT mice were challenged with epicutaneous PCl. Swelling was measured 24 h after challenge. Data represent the pooled results of three experiments and are the mean swelling ± SEM of 8–9 mice per group. *P < 0.03. NAIVE, non-immunized; IMM, immunized.
To more specifically test the hypothesis that sensitivity to TGF-β is required for the suppression of DTH in vivo by AC-SPL cells, spleen cells recovered from WT mice that had received an intracameral injection of TNP–BSA were tested for suppressive ability in the LTS in WT or dnTGFβRII mice. T cells in dnTGFβRII mice are largely resistant to the effects of TGF-β as a result of the T cell-specific expression of a dominant negative TGF-β receptor II (22). Although the PCl-induced footpad swelling responses were generally smaller in this series of experiments, the DTH responses were suppressed in the WT mice receiving the WT regulatory spleen cells while the responses were not significantly suppressed in the dnTGFβRII mice receiving the WT regulatory spleen cells (Fig. 5).
Fig. 5.
DTH in dnTGFβRII mice is resistant to suppression by WT-AC-SPL cells. Twenty-five thousand AC-SPL cells from C57BL/6 mice that received intracameral TNP–BSA were injected into footpads intradermally immediately after the footpads of TNP–BSA-immunized dnTGFβRII mice or WT mice were challenged with epicutaneous PCl. Swelling was measured 24 h after challenge. The data represent the pooled mean swelling ± SEM of 9–10 mice per group in three experiments. *P < 0.02. Immunized: immunized, challenged mice; NAIVE: swelling in challenged, non-immunized mice. NAIVE, non-immunized; IMM, immunized.
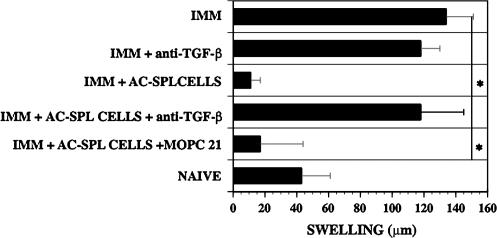
The suppression of the DTH reaction by CD8+ AC-SPL cells is inhibited by antibodies to TGF-β
Because CD8+ T cells in the spleens of mice that received an intracameral injection of antigen produce TGF-β (12, 23) and T cells resistant to TGF-β are resistant to suppression mediated by CD8+ AC-SPL cells, we reasoned that the DTH-suppressive mechanism of the CD8+ AC-SPL cells may be dependent on TGF-β. To test this hypothesis, antibodies that recognize TGF-β 1–3 were included in the inoculum of C57Bl/6 AC-SPL cells injected into footpads of TNP–BSA-immunized WT mice immediately after the challenge with epicutaneous PCl. The swelling of the footpads that received AC-SPL cells and control IgG1 antibody (MOPC 21) was suppressed significantly, i.e. ∼75% less swelling than that of challenged, immunized mice that did not receive regulatory spleen cells (Fig. 6). However, the swelling of the footpads that received AC-SPL cells and anti-TGF-β antibodies was not significantly different from that of challenged immunized mice that received PCl only or PCl and the isotype control antibody MOPC 21. Similar results were obtained when mice received unseparated AC-SPL cells ± anti-TGF-β antibody (data not shown).
Fig. 6.
Anti-TGF-β antibodies inhibit the suppressive activity of AC-SPL cells in the LTS. WT mice were immunized with TNP–BSA 1 week after they received an intracameral injection of TNP–BSA. One week after immunizing, spleen cell suspensions were prepared. The cell suspensions were incubated for 30 min at 4°C. in PBS, PBS + 15 μg anti-TGF-β 1–3 antibodies or control MOPC 21 IgG1 antibody. After incubation, the cells ± antibodies or anti-TGF-β antibody only were injected into footpads of TNP–BSA-immunized recipients immediately after the footpad received an epicutaneous challenge with PCl. Swelling was measured 24 h later. Data represent the mean swelling ± SEM of six mice per group pooled from two experiments. *P < 0.01. NAIVE, non-immunized; IMM, immunized.
Discussion
The injection of antigen into the AC generates circulating F4/80+ cells that migrate to the spleen and thymus (10, 24–26). Thymic NKT cells, activated by these F4/80+ cells migrate to the spleen where, in concert with F4/80+ cells, marginal zone B cells and splenic NKT cells activate regulatory CD8+ T cells (11). Whether these F4/80+ cells were circulating cells recruited to the AC and then recirculated or were resident in the iris is not known. However, TGF-β in aqueous humor and/or produced by iris F4/80+ cells induces a suppressive phenotype in F4/80+ cells (25). Therefore, we investigated whether TGF-β is required directly by T cells in vivo for the generation of splenic CD8+ regulatory T cells after the injection of antigen into the AC. Although unseparated dnTGFβRII and Cbl-b−/− AC-SPL cells did not suppress the DTH reaction when injected into the footpads of immunized mice immediately after antigenic challenge, CD8+ AC-SPL cells enriched from the unseparated cell population suppressed the DTH response (Fig. 3A and B). The unseparated cell population could contain CD4+-sensitized T cells resistant to TGF-β-mediated suppression that would transfer the DTH reaction to the sensitized recipients. Because Cbl-b−/− and dnTGFβRII mice produce CD8+ regulatory AC-SPL cells, the generation of circulating F4/80+ cells and regulatory thymocytes is also intact (data not shown). We have observed that the spleens of dnTGFβRII mice receiving intracameral antigen and immunization contain different numbers of DTH-inducing T cells and CD8+ regulatory T cells such that each cell population can be demonstrated when transferring higher or lower numbers of cells (R. E. Cone and Y. Lemire, unpublished observations). TGF-β can ‘convert’ activated CD8+ T cells to a suppressive phenotype in vitro (15). Therefore, our results do not preclude a direct role for TGF-β in the in vivo generation of CD8+ regulatory T cells after the intracameral injection of antigen. In particular, if some circulating CD8+ regulatory T cells migrate to the AC perhaps they will be ‘converted’ to regulatory T cells in the AC by TGF-β in the aqueous humor. However, F4/80+ cells in the AC likely have a suppressive phenotype due to the TGF-β in aqueous humor and are central to the induction of peripheral regulatory T cells.
CD8+ regulatory T cells, unlike CD4+, CD25+ FoxP3+ regulatory T cells suppress activated T cells (1–3, 7). Moreover, CD8+ regulatory T cells are detected after immunization and there is no evidence at present for naturally occurring CD8+ regulatory T cells. Unlike CD4+ Treg, the suppression effected by CD8+ Treg is highly antigen specific (1, 2) and may be directed toward TCR V-region epitopes (1, 5). Like CD4+ Treg, the suppressive mechanisms of CD8+ regulatory T cells have not been defined. This issue is complicated by the description of several types of CD8+ regulatory T cells that may suppress by distinct mechanisms (27–29). CD8+ regulatory T cells can be induced in vivo (1–6) or in vitro (15, 16). We chose to investigate a mechanism of suppression effected by CD8+ regulatory T cells induced in vivo because it is well established that the injection of antigen into the AC induces the production of antigen-specific, splenic CD8+ regulatory T cells that are capable of suppressing a DTH reaction in sensitized recipients (6, 8–12, 26, 30). We confirmed that the suppression of the DTH reaction in the LTS is specific to the antigen injected into the AC. CD8− or CD4+ AC-SPL cells do not suppress the DTH reaction in the LTS or the local adoptive transfer assay (8–10, 31). Therefore, it is unlikely that CD4/CD25+ T cells suppress the DTH reaction in the LTS. However, intracamerally induced CD4+ T cells induce CD8+ regulatory T cells (23, 25, 32). Splenic regulatory CD8+ T cells are induced in vitro by TGF-β or TGF-β, antigen-pulsed F4/80+ cells (15, 16). CD8+ regulatory T cells are induced (converted?) in vitro by TGF-β, the in vitro ligation of 4-1BB by anti-4-1BB antibodies (13, 14) or the injection of antigen into the AC (12, 23) produce TGF-β. Therefore, we asked whether TGF-β could be a mechanism used by CD8+ regulatory AC-SPL cells to mediate the suppression of DTH. We determined whether the DTH reaction is suppressed by CD8+ AC-SPL cells in the presence of antibodies to TGF-β or whether the DTH reaction in immunized mice with T cells resistant to TGF-β is suppressed by CD8+ AC-SPL cells in the LTS. Our observation of a complete inhibition in vivo of the suppression of DTH to PCI by TNP-specific–BSA-AC-SPL cells by antibodies to TGF-β isoforms 1−3 is similar to our observation of the anti-TGF-β-induced inhibition of the suppression of OVA-induced DTH by OVA-specific AC-SPL cells (33). The inhibition of CD8+ AC-SPL cell-mediated suppression of DTH is consistent with a report that the DTH reaction is enhanced by antibodies to anti-TGF-β injected into the immunized recipients at the site challenged with antigen to elicit a DTH reaction (34). Moreover, we observed that two strains of mice, Cbl-b−/− and dnTGFβRII, in which the T cells are resistant to TGF-β based on different mechanisms, are also resistant to the suppression of the DTH reaction by CD8+ AC-SPL cells. In one strain, dnTGFβRII, there is a proximal defect in TGF-β signaling in T cells resulting from the transgenic expression of a dnTGFβRII (17, 22). In the second strain, Cbl-b−/− mice have a normal receptor for TGF-β but the absence of the ubiquitin ligase Cbl-b renders the cells incapable of responding to TGF-β signaling (20, 21).
Although both strains may have immunological defects, we show that the DTH response to immunization is similar (or sometimes stronger) than that induced in a WT mouse. In these studies, we did not generally use isolated splenic CD8+ cell populations to suppress a local DTH reaction because we demonstrated that CD8+ but not CD4+ splenic regulatory T cells induced by the injection of antigen into the AC selectively suppress the in vivo DTH reaction (Fig. 1). Moreover, it is unlikely that the suppressive activity of the CD8+ regulatory spleen cells induced by intracameral antigen is mediated through cytotoxicity because in the LTS suppression of the DTH reaction by regulatory spleen cells is independent of perforin and FasL (9).
Mice deficient in Cbl-b have T cells that are CD28 independent in their activation and hyperactive in their responses (18–21). In addition, Cbl-b−/− mice develop spontaneous autoimmunity and have increased susceptibility to elicited autoimmunity (18, 19). Although Cbl-b−/− mice have normal numbers of functional CD4+CD25+ Foxp3+ regulatory T cells, their effector T cells are resistant to the suppression mediated by both TGF-β and CD4+CD25+ Treg cells (20). Cbl-b−/− effector T cells have been shown to express normal levels of TGF-βRII, though an abnormality in Cbl-b−/− T cells in the phosphorylation of Smad2 has been described (20). In the present studies, we extended these findings and demonstrate that Cbl-b−/− effector T cells are also resistant in vivo to CD8+ regulatory T cells.
Because the resistance of Cbl-b−/− mice to TGF-β is likely due to the complexities of signaling dysfunction in these mice, we also investigated TGF-β resistance in dnTGFβRII mice in which the resistance to TGF-β is a result of a dominant negative TGF-β receptor II expressed specifically on T cells (16, 22). As with DTH effector cells in Cbl-b−/− mice, DTH effector T cells in dnTGFβRII mice were resistant to suppression by splenic CD8+ regulatory spleen cells. That both strains, resistant to TGF-β by completely different mechanisms, are resistant to the suppression effected by CD8+ regulatory T cells suggests that the resistance of the two strains to suppression mediated by CD8+ regulatory T cells has a common basis.
CD8+ T (OT-1) cells converted to a suppressive phenotype by culture with TGF-β and OVA suppressed the induction of OVA-specific cytotoxic T cells in vitro and the rejection of a cardiac allograft in mice transgenic for OVA by a TGF-β-independent mechanism (15). Because the CD8+ regulatory T cells were induced by exposure to exogenous TGF-β and antigen in vitro, they may differ from some CD8+ regulatory T cells induced in vivo. Different subtypes of CD8+ regulatory T cells have been described that function by distinct non-lytic mechanisms (27, 28). Our results with antibodies to TGF-β and the resistance of TGF-β-resistant mice to the suppression of the DTH reaction by CD8+ AC-SPL cells provide evidence that TGF-β is an efferent suppressive mechanism of these cells. However, the LTS assay measures the suppression of DTH by a limited number of cells. It is possible that the use of larger numbers of cells may reveal additional suppressive mechanisms. Additionally, T cell-derived TGF-β1 has pleiotropic effects and may promote Th17 cell differentiation while suppressing Th1 cell differentiation (35, 36). Our experiments have not identified the TGF-β isoforms responsible for immunosuppression by CD8+ regulatory T cells nor the induction of AC-induced F4/80+ T cells required to induce immunoregulatory thymocytes or splenic CD8+ T cells. Accordingly, the afferent and efferent roles for TGF-β in the suppression of cell-mediated immunity induced by the intracameral injection of antigen are likely dependent on the complexity of the phenomenon under investigation.
Funding
EY017289 and EY017537 (to R.E.C.); National Institutes of Health (1R56 AI 072533 to R.B.C.); Connecticut Lions Eye Research Foundation to J.O.R.
Glossary
Abbreviations
- AC
anterior chamber
- CS
contact sensitivity
- dnTGFβRII
dominant negative receptor for transforming growth factor β receptor type II
- DTH
delayed-type hypersensitivity
- LTS
local transfer of suppression
- OVA
ovalbumin
- PCl
picryl chloride
- TGF
transforming growth factor
- TNP
trinitrophenol
- WT
wild type
References
- 1.Jiang H, Chess L. An integrated model of immunoregulation mediated by regulatory T cell subsets. Adv. Immunol. 2004;83:253. doi: 10.1016/S0065-2776(04)83008-6. [DOI] [PubMed] [Google Scholar]
- 2.Cantor H. Reliving suppression? Nat. Immunol. 2004;5:347. doi: 10.1038/ni0404-347. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Flood PM, Gershon RK. Immunoregulatory T cell pathways. Ann. Rev. Immunol. 1983;1:439. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc. Natl Acad. Sci. USA. 2001;98:6301. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang X, Maricic I, Purohit N, et al. Regulation of immunity by a novel population of Qa-1-restricted CD8αα+TCR α,β+ T cells. J. Immunol. 2006;177:7645. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- 6.Faunce DE, Terajewicz A, Stein-Streilein J. In vitro generated tolerogenic APC induce CD8+ T regulatory cells that can suppress ongoing experimental autoimmune encephalomyelitis. J. Immunol. 2004;172:1991. doi: 10.4049/jimmunol.172.4.1991. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Brook MO, Carvalho-Caspar M, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl Acad. Sci. USA. 2007;104:19954. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen, evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunol. 1990;71:383. [PMC free article] [PubMed] [Google Scholar]
- 9.Cone RE, Li X, Sharafieh R, O'Rourke J, Vella AT. The suppression of delayed-type hypersensitivity by CD8+ regulatory T cells requires interferon-γ. Immunol. 2006;120:112. doi: 10.1111/j.1365-2567.2006.02486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Wang Y, Urso D, O'Rourke J, Cone RE. Thymocytes induced by antigen injection into the anterior chamber activate splenic CD8+ suppressor cells and enhance the antigen-induced production of immunoglobulin G1 antibodies. Immunol. 2004;113:44. doi: 10.1111/j.1365-2567.2004.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niederkorn JY. Regulatory T cells and the eye. Chem. Immunol. Allergy. 2007;92:131. doi: 10.1159/000099263. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Ghali WE, Pingle P, et al. Splenic T cells from mice receiving intracameral antigen suppress in vitro antigen-induced proliferation and interferon gamma production by sensitized lymph node cells. Ocular Immunol. Inflamm. 2003;11:39. doi: 10.1076/ocii.11.1.39.15578. [DOI] [PubMed] [Google Scholar]
- 13.Myers L, Croft B, Kwon BS, Mittler RS, Vella AT. Peptide-specific CD8 regulatory cells use IFN-γ to elaborate TGF-β suppression. J. Immunol. 2003;174:7625. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- 14.Menoret A, Myers LM, Lee S-J, Mittler RS, Rossi RJ, Vella AT. TGF-β protein processing and activity through TCR triggering of primary CD8+ T regulatory cells. J. Immunol. 2006;177:6091. doi: 10.4049/jimmunol.177.9.6091. [DOI] [PubMed] [Google Scholar]
- 15.Kapp JA, Honjo K, Kapp LM, Xu XY, Cozier A, Pat Bucy R. TCR transgenic CD8+ T cells activated in the presence of TGF-ß express FoxP3 and mediate linked suppression of primary immune responses and cardiac allograft rejection. Int. Immunol. 2006;18:1549. doi: 10.1093/intimm/dxl088. [DOI] [PubMed] [Google Scholar]
- 16.Kezuka T, Streilein JW. In vitro generation of regulatory CD8+ T cells similar to those found in mice with anterior chamber-associated immune deviation. Invest. Ophthalmol. Vis. Sci. 2000;41:1803. [PubMed] [Google Scholar]
- 17.Wan YY, Flavell RA. Yin-Yang functions of transforming growth factor β and T regulatory cells in immune regulation. Immunol. Rev. 2007;222:199. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachmaier K, Krqwczyk C, Kozieradzki I, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 19.Chiang YJ, Kole HK, Brown K, et al. Cbl-b regulates CD28 dependence of T cell activation. Nature. 2000;403:216. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 20.Wohlfert EA, Callahan MK, Clark RB. Resistance To CD4+,CD25+ regulatory T cells and TGF-β in Cbl-b -/- mice. J. Immunol. 2004;173:1059. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 21.Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo. J. Immunol. 2006;176:1316. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- 22.Gorelik L, Flavell RA. Abrogation of TGF-β signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 23.Jiang L, He H, Yang, Lin P, et al. Splenic CD8+ T cells secrete TGF-β1 to exert suppression in mice with anterior chamber-associated immune deviation. Graefes archive for experimental and clinical ophthalmology. On Line First: October no. 8. 2008 doi: 10.1007/s00417-008-0947-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Goldschneider I, O'Rourke J, Cone RE. Blood mononuclear cells induce regulatory NK thymocytes in anterior chamber-associated immune deviation. J. Leukoc. Biol. 2001;69:741. [PubMed] [Google Scholar]
- 25.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID) II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J. Immunol. 1991;146:3018. [PubMed] [Google Scholar]
- 26.Faunce DE, Sonoda K-H, Stein-Streilein J. MIP-2 mediated recruitment of NKT cells to the spleen during tolerance induction. J. Immunol. 2001;166:313. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- 27.Xystrajus E, Dejean AS, Bernard I, et al. Identification of a novel CD8 T cell subset and analysis of its mechanism of regulation. Blood. 2004;104:3294. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 28.Gilliet M, Liu MYJ. Generation of human CD8 regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 2002;195:695. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang XL, Smith R, Kumar RV. Specific control of immunity by regulatory CD8 T cells. Cell Mol. Immunol. 2006;2:11. [PubMed] [Google Scholar]
- 30.D'Orazio TG, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the production of anterior chamber-associated immune deviation. Immunol. 1998;95:47. doi: 10.1046/j.1365-2567.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonoda K-H, Exley M, Snapper S, Balk ST, Stein-Streilein J. CD1-reactive natural killer T cells are required for the development of systemic tolerance through an immune privileged site. J. Exp. Med. 1999;190:1215. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skelsey ME, Mayhew E, Niederkorn JY. CD25+, interleukin-10-producing CD4+ T cells are required for suppressor cell production and immune privilege in the anterior chamber of the eye. Immunol. 2003;110:18. doi: 10.1046/j.1365-2567.2003.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chattopadhyay S, O'Rourke J, Cone JRE. Implication for the CD94/NKG2A-Qa-1 system in the generation and function of ocular-induced splenic CD8+ regulatrory T cells. Intern. Immunol. 2008;20:509. doi: 10.1093/intimm/dxn008. [DOI] [PubMed] [Google Scholar]
- 34.Bickerstaff AA, Xia D, Pelletier RP, Orosz CG. Mechanisms of graft acceptance: evidence that plasminogen activator controls donor-reactive delayed-type hypersensitivity responses in cardiac allograft acceptor mice. J. Immunol. 2000;164:5132. doi: 10.4049/jimmunol.164.10.5132. [DOI] [PubMed] [Google Scholar]
- 35.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor β and interleukin 10. Immunity. 2008;28:468. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1-and Th17-cell differentiation. Immunity. 2007;26:579. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]