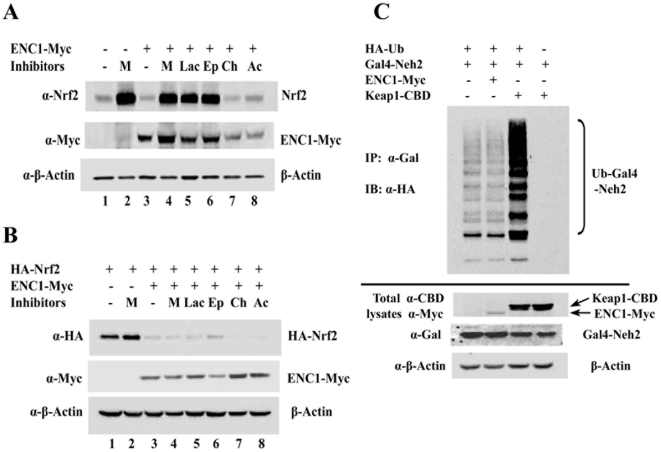
Figure 3. ENC1-mediated down-regulation of Nrf2 was independent of proteasomal or lysosomal degradation.
A. MDA-MB-231 cells were transfected with an empty vector or a vector for ENC1-Myc. Transfected cells were treated with proteasome inhibitors MG132 (M) (10 µM), clasto-lactacystin β-lactone (Lac) (10 µM) or epoxomicin (Ep) (1 µM), or lysosome inhibitors chloroquine (Ch) (50 µM) or ammonium chloride (Ac) (50 mM) for 4 hr before cells were lysed at 48 hr post-transfection. Cell lysates were subjected to immunoblot analysis with anti-HA, anti-Myc and anti-β-actin antibodies. B. MDA-MB-231 cells were transfected with HA-Nrf2 and with an empty vector or ENC1-Myc. Transfected cells were treated with proteasome and lysosome inhibitors as described above. Immunoblot analysis was performed. C. In vivo ubiquitination assay was performed in MDA-MB-231 cells transfected with plasmids for HA-Ub and Gal4-Neh2, along with either ENC1-Myc or Keap1-CBD. Transfected cells were treated with 10 µM MG132 for 4 hr prior to cell lysis. Cell lysates were denatured by heating and subjected to immunoprecipitation with anti-Gal4 antibodies. The precipitated protein complexes were subjected to immunoblot analysis with anti-HA antibodies for detecting ubiquitin-conjugated Gal4-Neh2 (top panel). Small aliquots of total cell lysates were immunoblotted with the indicated antibodies (bottom three panels).