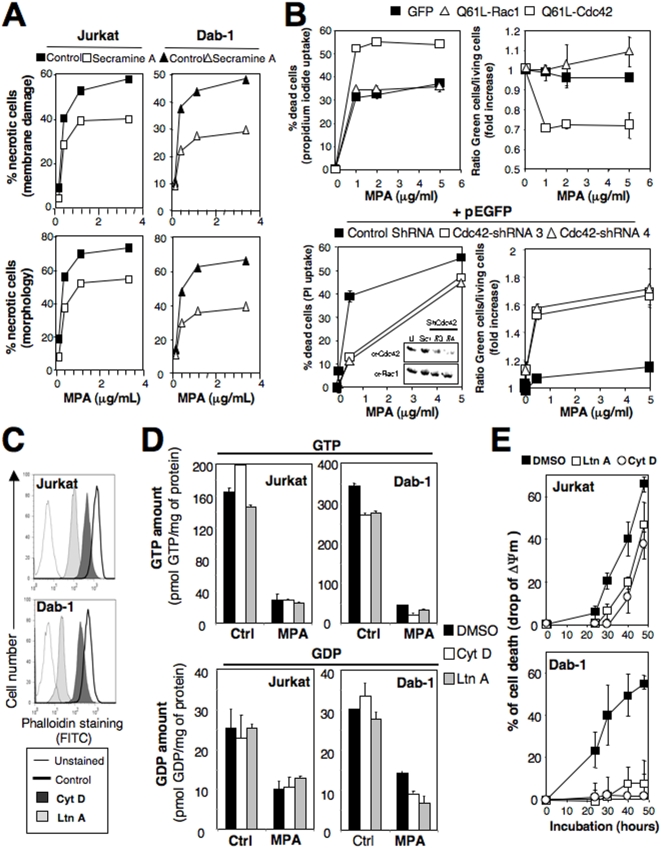
Figure 2. Cdc42 and actin are implicated in the MPA-mediated necrotic signal.
(A) Cells were pre-incubated with secramine A (40 µM) or DMSO for 1 hour and then incubated for 44 hours with MPA. Necrosis was quantified by assessing the loss of the plasma membrane integrity (entry of propidium iodide) and the cell morphology. (B) Upper panel: Jurkat cells transfected with plasmid encoding Q61L-Cdc42-GFP, Q61L-Rac1-GFP or GFP alone were treated with MPA for 44 hours. The percentage of dead cells (morphologic analysis) and the percentage of GFP-expressing cells among the living population are shown. Lower panel: Jurkat cells were transfected with the Cdc42-targeting shRNA or control shRNA (Scr) and pEGFP-N1 at a DNA weight ratio of 3∶1. 24 hours after transfection, living cells were isolated by Ficoll gradient and incubated for 44 hours with MPA. Cell death was assessed by analyzing plasma membrane damage (propidium iodide entry). The inset depicts the expression of Cdc42 and Rac1 in Jurkat cells untransfected (U) or transfected with scramble shRNA (Scr) or shRNA targeting Cdc42. Western blots were performed using the indicated antibodies. (C) The B-cell line Dab-1 and the T-cell line Jurkat were treated with Latrunculin (LtnA, 2.5 µM), Cytochalasin D (CytD, 5 µM) or DMSO (control) for 30 minutes. Cells were then fixed, permeabilized and F-actin was tagged using FITC-labeled phalloidin. The amount of F-actin was assessed by flow cytometry. (D) Cells were pre-incubated for 30 min with 2.5 µM of LtnA, 5 µM of CytD or DMSO and then treated in presence or not of MPA (5 µg/ml) for 14 hours. The amount of GTP (upper panel) and GDP (lower panel) was quantified as described in Materials and Methods. (E) Cells were pre-incubated for 30 min with LtnA (2.5 µM) or CytD (5 µM) and then treated with 5 µg/ml of MPA for the indicated times. ΔΨm was assessed using DiOC6 staining. Data represent the mean±SD of three independently performed experiments.