Fig. 4.
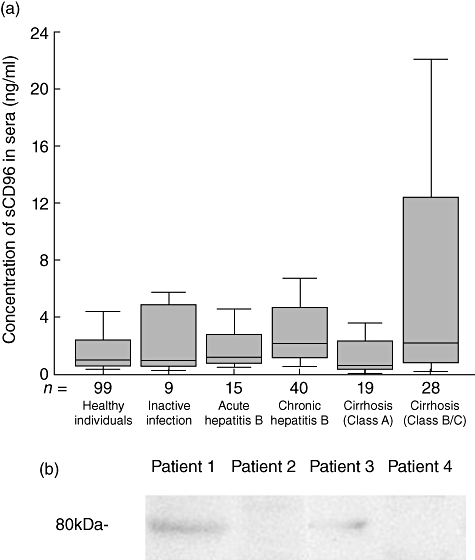
Detecting soluble CD96 in sera by the enzyme-linked immunosorbent assay (ELISA) kit and Western blot analysis for sCD96 in patients’ sera. (a) The level of soluble CD96 in sera from healthy individuals with or without inactive hepatitis B virus (HBV) infection, patients with acute and chronic viral hepatitis B and patients with hepatic cirrhosis at different stages (outliers and extremes were not displayed in the box plot but were involved in the statistical analysis). The bottom of each box represents the 25th percentile, the top is the 75th percentile, and the line in the middle represents the median. Upper adjacent represents maximum excluding outliers and extremes. Lower adjacent represents minimum. (b) Western blot analysis for sCD96 in patients’ sera. Lane 1, a patient with viral hepatitis B (sCD96, 396 ng/ml); lane 2, a patient with viral hepatitis B (sCD96, 3·65 ng/ml); lane 3, a patient with hepatic cirrhosis (sCD96, 181 ng/ml); lane 4, a patient with hepatic cirrhosis (sCD96, 3·95 ng/ml).
