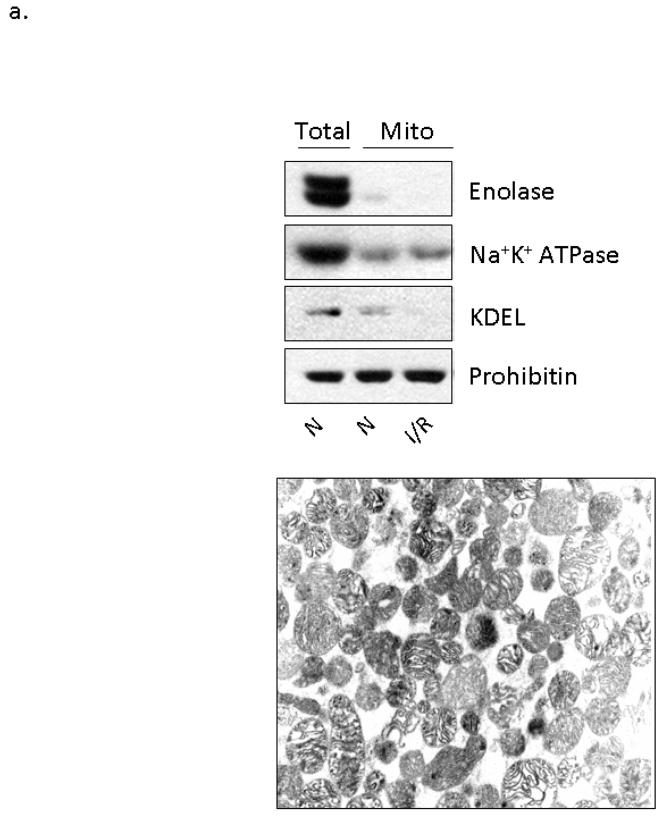
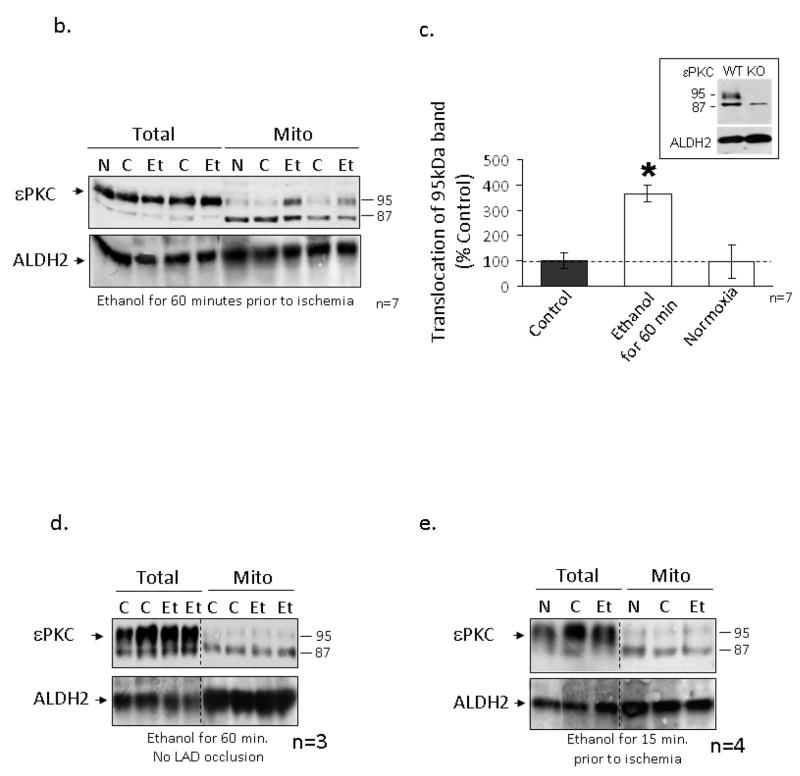
Figure 2. Ethanol-mediated protection is associated with εPKC translocation to cardiac mitochondria.
Mitochondria were isolated from the left ventricles of animals that were treated with 0.5 g/kg of ethanol 15 and 60 minutes prior to LAD occlusion (Et) and compared to animals that were not treated with ethanol (C) and to animals that were not subjected to LAD occlusion (N). Following homogenization, mitochondrial lysates and total cellular lysates were analyzed by Western blot analysis utilizing an anti-εPKC antibody and equal loading was determined by monitoring the levels of mitochondrial ALDH2. (a) Electron micrographs illustrating the membrane integrity of isolated mitochondria. Additionally, the presence of mitochondrial protein (prohibitin), and the lack of cytosolic proteins (enolase), plasma membrane proteins (Na+K+ ATPase), and ER resident proteins (KDEL) illustrates the purity of our mitochondrial preparation. (b) Data from two separate groups of animals treated with ethanol 60 minutes prior to LAD ligation are shown. Two different molecular weight forms of mitochondrial εPKC are marked with arrows. (c) Quantification of εPKC translocation to the mitochondria from 7 different experiments was done using NIH Image-J software, expressed as % control and significance was determined with a 2 way t-test (*= p<0.05). Significant changes were observed between the ethanol treated and control and normoxic hearts. (1c Insert) Hearts from wildtype and εPKC knock-out mice were isolated and homogenized for Western blot analysis. The lower 87kDa band is present in both wildtype and knock-out animals suggesting non-specific antibody recognition while the upper band represents εPKC. (d) Animals were subjected to ethanol treatment without LAD occlusion and data from two representative experiments are shown. (e) Representative data from 4 separate experiments from animals treated with ethanol 15 minutes prior to LAD ligation are shown.