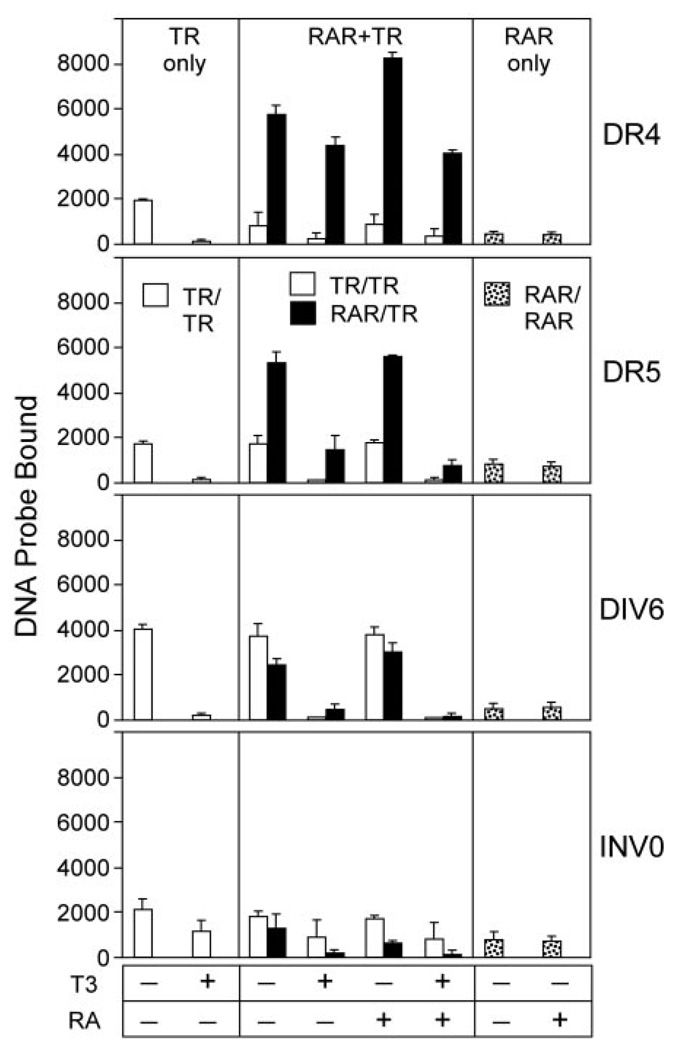
Fig. 2. RARα/TRα Heterodimers Form on a Series of Prototypic DNA Response Elements.
The ability of RARα and TRα to bind as homodimers and as heterodimers to a variety of DNA response elements was tested using an EMSA protocol as in Fig. 1. Radiolabeled DR-4, DR-5, DIV-6, and INV-0 DNA elements, all composed of AGGTCA half-site repeats, were tested. TRα and RARα proteins, used at 40 and 28 ng respectively, were assayed alone (left and right panels), or as an RARα/TRα mixture (center panels). Where indicated, T3 or ATRA was also included at 1 µM in the EMSA binding buffer. The amount of radiolabeled DNA probe migrating as a TRα homodimer (open bars), RARα homodimer (stippled bars), or RARα/TRα heterodimer (filled bars) under each condition was quantified; the mean and se of three experiments are shown.