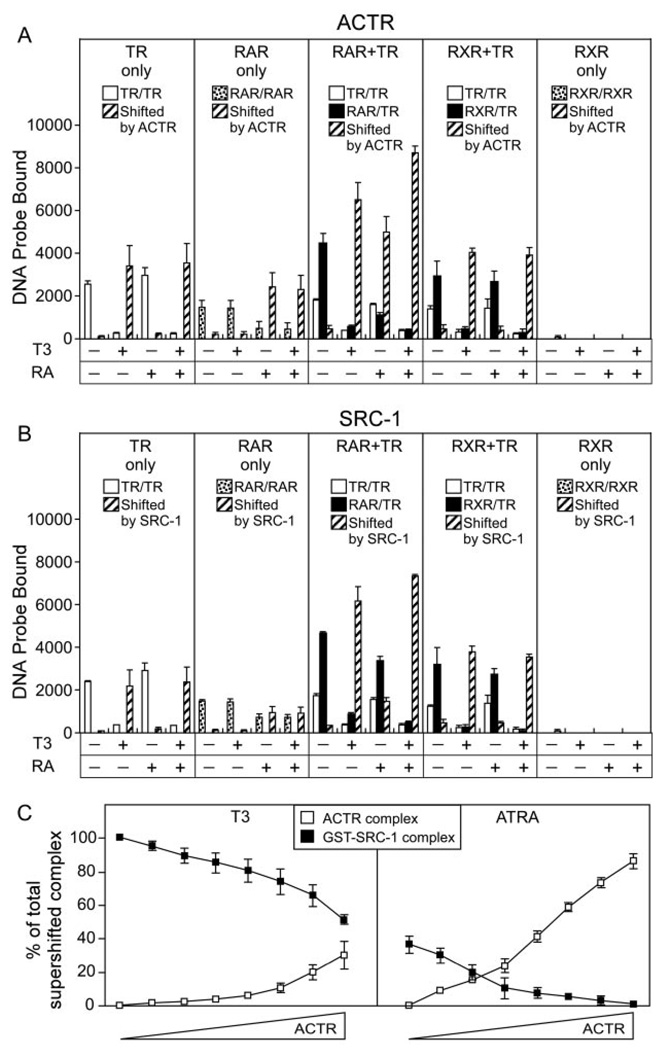
Fig. 8. Coactivators Are Recruited by RARα/TRα Heterodimers in Response to Cognate Hormones.
The ability of various TR, RAR, and RXR complexes to bind ACTR coactivator in response to different hormones was determined. A, TRα homodimers (TR only), RARα homodimers (RAR only), RXRγ homodimers (RXR only), RARα/TRα heterodimers (RAR + TR), and RXRγ/TRα heterodimers (RXR + TR) were assembled on a radiolabeled DR-4 DNA probe in the presence of a GST-ACTR coactivator construct. T3 alone, ATRA alone, 9-cis retinoic acid, or T3 + ATRA was included in the binding assay at 1 µM concentrations as indicated below the panels. The resulting protein/DNA complexes were resolved by EMSA. The amount of radiolabeled probe migrating as a TRα (open bars), RARα, or RXRγ homodimer (stippled bars); as an RAR/TR or RXR/TR heterodimer (filled bars); or as an ACTR supershifted receptor/DNA complex (crosshatched bars) was quantified under each condition and is presented. TRα, RARα, RXRγ, and GST-ACTR were used at 40 ng, 21 ng, 7 ng, and 1 µg per assay respectively. B, The same experiment as in panel A was performed employing a GST-SRC-1 coactivator construct (1 µg per assay). C, The EMSA supershift experiments in A and B were repeated using a competition strategy. RARβ/TRα heterodimers, assembled on a DR-4 DNA probe, were mixed with 2 µg of the SRC-1 construct either alone or with increasing amounts (0.0156 to 1 µg in 2-fold increments) of the ACTR construct; the experiment was performed in the presence of 1 µM T3 or ATRA, as indicated. The amount of RARβ/TRα/DNA complex supershifted into a SRC-1 or into an ACTR complex was determined for each ACTR concentration as for panels A and B. The mean and se of three experiments are shown.