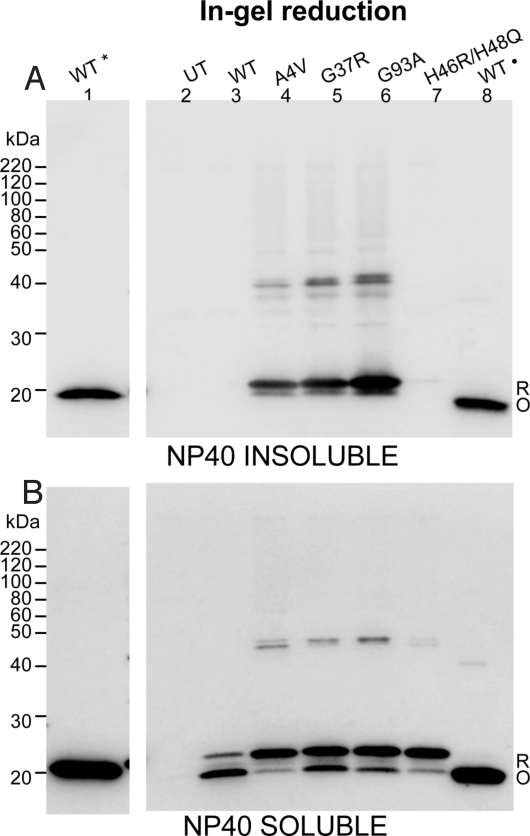
Fig. 4.
Reduced hSOD1 protein is preferentially incorporated into detergent-insoluble aggregates. HEK293FT cells were transfected with vectors for WT, A4V, G37R, G93A, and H46R/H48Q hSOD1 proteins, harvested after 48 h, and extracted in buffers with 0.5% Nonidet P-40 and 100 mM IA (representative experiment from 4 repetitions). Detergent-insoluble (A) and soluble fractions (B) from cells transfected with each construct were electrophoresed in the absence of βME (lanes 2–8). Lane 1 of each panel contains purified WT SOD1 that was reduced before electrophoresis (*). This lane was separated from the remaining samples by a lane containing marker proteins and an empty space to prevent reducing agent from diffusing into other samples (these intervening lanes have been cropped out of the gel image). Lane 8 of each panel contains purified WT SOD1 that was verified to have in intact intramolecular disulfide bond (·). Purified WT SOD1 proteins were treated with 100 mM IA prior boiling in Sample buffer and electrophoresis. To enhance detection of oxidized forms of hSOD1, gels were incubated in transfer buffer containing 2% βME for 10 min before electrotransfer to nitrocellulose membranes. UT, untransfected cells; R, reduced disulfide bond (C57-C146). O, oxidized disulfide bond (C57-C146).