Table 1. Summary of data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Zinc-bound crystal | |||||
|---|---|---|---|---|---|
| 1st crystal | |||||
| Zn edge | Zn peak | Zn remote | 2nd crystal | Zinc-free iron-bound crystal | |
| Data collection | |||||
| Wavelength (Å) | 1.2829 | 1.2822 | 1.0000 | 1.0000 | 1.0000 |
| Resolution range (Å) | 50.0–2.40 (2.49–2.40) | 50.0–2.20 (2.28–2.20) | 50.0–2.80 (2.90–2.80) | ||
| No. of observed reflections | 125496 | 136427 | 139258 | 125281 | 69903 |
| No. of unique reflections | 36071 | 37010 | 37531 | 26551 | 12243 |
| Data completeness (%) | 96.6 (74.3) | 99.9 (99.3) | 100.0 (100.0) | 99.3 (98.4) | 99.8 (100.0) |
| Rmerge† | 0.040 (0.384) | 0.041 (0.256) | 0.040 (0.239) | 0.063 (0.272) | 0.096 (0.148) |
| 〈I〉/〈〈σ(I)〉 | 15.5 (1.3) | 19.0 (2.7) | 20.8 (2.9) | 9.1 (2.1) | 51.1 (23.3) |
| Space group | I222 | P31 | |||
| Unit-cell parameters (Å, °) | a = 79.1, b = 114.1, c = 114.7 | a = b = 78.6, c = 71.9, α = β = 90, γ = 120 | |||
| Refinement | |||||
| Resolution range used for refinement (Å) | 50.0–2.20 | 20.0–2.80 | |||
| Rfactor‡ (%) | 18.8 | 26.7 | |||
| Rfree‡ (%) | 22.9 | 29.9 | |||
| No. of reflections used for refinement | 24819 | 12171 | |||
| Protein residues modelled | 406 of 414 | 397 of 414 | |||
| No. of protein atoms modelled | 3061 | 3019 | |||
| No. of water molecules modelled | 158 | 16 | |||
| Mean overall B value (Å2) | 16.3 | 42.0 | |||
| R.m.s.d. bond angle (°) | 1.225 | 0.895 | |||
| R.m.s.d. bond length (Å) | 0.010 | 0.005 | |||
| Ramachandran plot | |||||
| Residues in most favoured regions (%) | 85.5 | 82.4 | |||
| Residues in additionally allowed regions (%) | 14.5 | 17.3 | |||
| Residues in generously allowed regions (%) | 0.0 | 0.3 | |||
| Residues in disallowed regions (%) | 0.0 | 0.0 | |||
R
merge = 
 .
.
R
factor = 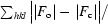
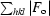 . R
free was calculated using 5% of the data that were excluded from refinement.
. R
free was calculated using 5% of the data that were excluded from refinement.
