Abstract
Objective
To examine the interaction between cigarette smoking and clinical efficacy of clopidogrel in STEMI.
Background
Cigarette smoking induces CYP1A2, which converts clopidogrel into its active metabolite, and prior studies suggest greater inhibition of platelet aggregation by clopidogrel in smokers of ≥10 cigarettes/day.
Methods
The effect of clopidogrel compared with placebo on angiographic and clinical outcomes was examined in CLARITY-TIMI 28, a randomized trial of 3429 STEMI patients, stratified by smoking intensity as follows: not current smokers (N=1732), smokers of 1-9 (N=206), 10-19 (N=354), 20-29 (N=715), and ≥30 cigarettes/day (N=422). Logistic regression was used to adjust for other baseline characteristics and interaction terms to test for effect modification.
Results
Although clopidogrel reduced the rate of the primary endpoint of a closed infarctrelated artery or death/MI before angiography in CLARITY-TIMI 28, the benefit was especially marked among those who smoked ≥10 cigarettes/day (adjusted OR 0.49, 95% CI 0.37-0.66; P<0.0001) as compared to those who did not (adjusted OR 0.72, 95% CI 0.57-0.91; P=0.006; Pinteraction=0.04). Similarly, clopidogrel was significantly more effective at reducing the rate of cardiovascular death, MI, or urgent revascularization through 30 days among those who smoked ≥10 cigarettes/day (adjusted OR 0.54, 95% CI 0.38-0.76; P=0.0004) compared with those who did not (adjusted OR 0.98; 95% CI 0.75-1.28; P=0.87; Pinteraction=0.006).
Conclusions
Cigarette smoking appears to positively modify the beneficial effect of clopidogrel on angiographic and clinical outcomes. This study demonstrates that common clinical factors that influence the metabolism of clopidogrel may impact its clinical effectiveness.
Keywords: clopidogrel, smoking, cytochrome P450
Clopidogrel is an oral thienopyridine inhibitor of the platelet P2Y12 adenosine diphosphate (ADP) receptor that has been shown to prevent death and adverse cardiovascular events in patients with acute coronary syndromes (1,2). However, there is substantial inter-patient variability in the response to clopidogrel (3), and several studies have demonstrated that a diminished response to clopidogrel is associated with an increased risk of ischemic events (4-7).
Clopidogrel is a pro-drug that requires two-step oxidization by cytochrome P450 (CYP) enzymes to be transformed into its active metabolite, 2-oxoclopidogrel, with CYP1A2 and CYP3A4 being important for the first and second steps, respectively (8). Genetic and environmental influences on CYP450 enzyme activity are thought to underlie the substantial inter-patient variability in the response to clopidogrel (9,10). Cigarette smoking is a known inducer of CYP1A2 (11) and therefore potentially capable of affecting the pharmacokinetics and pharmacodynamics of clopidogrel.
To that end, a recent study published in the Journal of the American College of Cardiology showed that cigarette smoking was associated with increased platelet inhibition and lower aggregation in response to clopidogrel (12). However, the clinical significance of these observations remains undefined. We therefore examined the interaction between cigarette smoking and the efficacy of clopidogrel therapy in a randomized clinical trial of clopidogrel in patients with an acute coronary syndrome.
Methods
Study Population
The design and primary results of CLARITY-TIMI 28 have been published (2,13). In brief, 3491 patients with ST elevation myocardial infarction (STEMI) who presented within 12 hours of symptom onset were to receive aspirin, a fibrinolytic, and heparin (required if they were to receive a fibrin-specific lytic), and were randomized to clopidogrel (300 mg loading dose followed by 75 mg daily) or placebo. As part of the trial protocol, patients were scheduled to undergo coronary angiography 2-8 days after initiation of therapy to assess for late patency of the infarct-related artery. Patients were followed for clinical outcomes and adverse events through 30 days following randomization. Smoking status and number of cigarettes smoked per day at baseline were collected on the case report form. The protocol was approved by the relevant institutional review boards, and written informed consent was obtained from all patients.
Outcomes
The primary efficacy endpoint was the composite of TIMI flow grade (TFG) 0 or 1, or death or recurrent myocardial infarction before angiography could be performed. The 30-day clinical endpoint was a composite of death from cardiovascular causes, recurrent MI, or recurrent ischemia leading to the need for urgent revascularization. Angiographic outcomes were assessed by the TIMI Angiographic Core Laboratory, and all ischemic events were adjudicated by a Clinical Events Committee, and both were blinded to the assigned treatment arm. Safety endpoints included TIMI major and minor bleeding.
Statistical Analysis
Patients were stratified into 5 groups by smoking intensity: non-current smokers, those who smoked 1-9 cigarettes/day, 10-19 cigarettes/day, 20-29 cigarettes/day, and ≥30 cigarettes/day. Baseline characteristics across groups were compared using Student t tests for normally distributed continuous variables, Wilcoxon rank sum tests for non-normally distributed continuous variables, and chi-square tests for categorical variables. Based on published data examining the intensity of smoking and the pharmacologic response to clopidogrel (12), we also applied a dichotomous cut point of smoking ≥10 cigarettes (1/2 pack) a day or not. The odds ratios (ORs) and 95% confidence intervals (CIs) for the effect of clopidogrel on the outcomes were calculated within each smoking intensity stratum in a logistic regression model that adjusted for age, sex, region of enrollment (based on the United Nations Statistics Geographic Region Codes), hypertension, diabetes, infarct location, time to fibrinolytic therapy, and type of fibrinolytic. Interaction terms in logistic regression models were used to test for the statistical significance of effect modification by smoking on the efficacy of clopidogrel (modeled as: smoking ≥10 cigarettes × randomization to clopidogrel).
Results
A total of 3491 patients underwent randomization in CLARITY-TIMI 28 and smoking status was available for 3429 patients. 1732 patients were not current smokers and 1697 were, the latter consisting of 206 patients who smoked 1-9 cigarettes/day, 354 who smoked 10-19 cigarettes/day, 715 who smoked 20-29 cigarettes/day, and 422 who smoked ≥30 cigarettes/day. Compared with non-smokers, smokers were younger, more likely to be male, less likely to have a history of hypertension or diabetes, and less likely to present with an anterior MI or get a fibrin-specific fibrinolytic (Table 1).
Table 1.
Baseline Characteristics of the Patients
| Variable | Non-current Smokers | Cigarettes per Day | ||||
|---|---|---|---|---|---|---|
| 0-9 | 10-19 | 20-29 | ≥30 | P-value | ||
| Number | 1732 | 206 | 354 | 715 | 422 | |
| Cigarettes per day, median (IQR) | n/a | 5 (2-6) | 10 (10-15) | 20 (20-20) | 40 (30-40) | n/a |
| Age, years±SD | 61.1±9.6 | 58.3±10.45 | 54.5±10.5 | 53.1±9.2 | 52.0±8.3 | <.0001 |
| Male sex, % | 77.0 | 81.1 | 72.6 | 83.9 | 93.6 | <.0001 |
| Region, % | ||||||
| North America and Northern and Western Europe | 53.2 | 47.1 | 55.1 | 46.9 | 40.3 | <.0001 |
| Southern and Eastern Europe | 24.5 | 26.7 | 22.0 | 29.0 | 32.7 | 0.0007 |
| Latin America | 13.6 | 18.4 | 13.8 | 13.0 | 13.0 | 0.5855 |
| Middle East and Australasia | 8.7 | 7.8 | 9.0 | 11.2 | 14.0 | 0.0009 |
| Hypertension, % | 50.8 | 45.6 | 40.9 | 34.7 | 33.3 | <.0001 |
| Hyperlipidemia, % | 41.3 | 34.1 | 38.3 | 35.9 | 35.1 | 0.0442 |
| Diabetes mellitus, % | 20.7 | 18.1 | 9.5 | 11.9 | 14.5 | <.0001 |
| Killip Class II-IV, % | 7.4 | 9.3 | 7.9 | 9.8 | 6.9 | 0.2726 |
| Anterior MI, % | 44.7 | 41.3 | 35.3 | 36.8 | 35.8 | <.0001 |
| Time from symptom onset to start of fibrinolytic, hours (IQR) | 2.7 (1.8-4.2) | 2.9 (2.0-4.4) | 2.6 (1.6-3.8) | 2.7 (1.8-4.2) | 2.5 (1.7-3.8) | 0.2353 |
| Fibrin-specific lytic, % | 70.6 | 68.4 | 68.4 | 61.8 | 70.4 | 0.0009 |
| Initial heparin, % | ||||||
| Unfractionated heparin | 46.2 | 45.6 | 43.2 | 42.8 | 50.0 | 0.1581 |
| Low-molecular-weight heparin | 30.1 | 30.1 | 30.2 | 28.1 | 30.1 | 0.8917 |
| Both | 4.8 | 3.9 | 6.5 | 5.2 | 3.8 | 0.4508 |
| Neither | 18.9 | 20.4 | 20.1 | 23.9 | 16.1 | 0.0158 |
| Concurrent administration of a CYP3A4-metabolized statin*, % | 60.6 | 57.6 | 58.5 | 60.6 | 60.0 | 0.8413 |
Includes atorvastatin, simvastatin, and lovastatin.
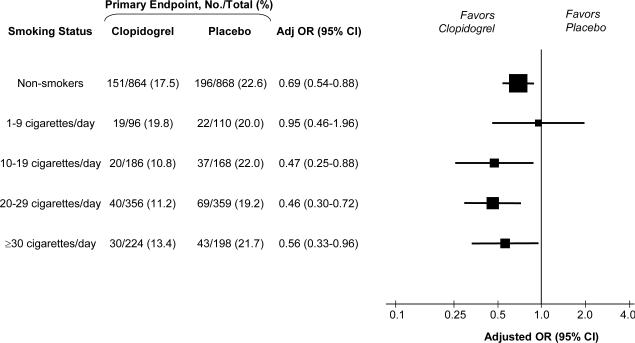
The effect of clopidogrel on the risk of the primary endpoint of a closed (TIMI flow grade 0 or 1) infarct-related artery or death or recurrent myocardial infarction prior to angiography stratified by intensity of smoking is shown in Figure 1. Overall in the trial, clopidogrel reduced the odds of the primary endpoint by 36% (OR 0.64, 95% CI 0.53-0.76, P<0.001). Among non-smokers or those who smoked less than half a pack per day, the addition of clopidogrel reduced the rate of the primary endpoint from 22.3% to 17.7%, with an adjusted OR of 0.72 (95% CI 0.57-0.91; P=0.006). However, among those who smoked half a pack a day or more, the addition of clopidogrel resulted in a far greater reduction in the rate of the primary endpoint from 20.5% to 11.7%, with an adjusted OR of 0.49 (95% CI 0.37-0.66; P<0.0001). A test for interaction between smoking, clopidogrel treatment, and the primary efficacy endpoint was significant (P=0.04), indicating a significantly greater benefit of clopidogrel in those who smoked at least half a pack per day. Furthermore, examining the likelihood of achieving optimal epicardial flow (TFG 3), treatment with clopidogrel resulted in an adjusted OR of 1.14 (95% CI 0.94-1.39; P=0.18) among non-smokers or those who smoked less than half a pack per day versus 1.77 (95% CI 1.40-2.22; P<0.0001) among those who smoked half a pack a day or more (Pinteraction=0.007), again demonstrating effect modification by smoking on clopidogrel.
Figure 1. Benefit of Clopidogrel on Primary Endpoint by Smoking Status.
The primary efficacy endpoint was a composite of Thrombolysis in Myocardial Infarction (TIMI) flow grade 0 or 1 on angiography or death or recurrent myocardial infarction prior to angiography. Odds ratios (OR) are adjusted for age, sex, region, hypertension, diabetes, infarct location, time to fibrinolytic therapy, and type of fibrinolytic. For each subgroup, the size of the box is proportional to the number of individuals included in the analysis. The horizontal lines represent the 95 percent confidence intervals (CI).
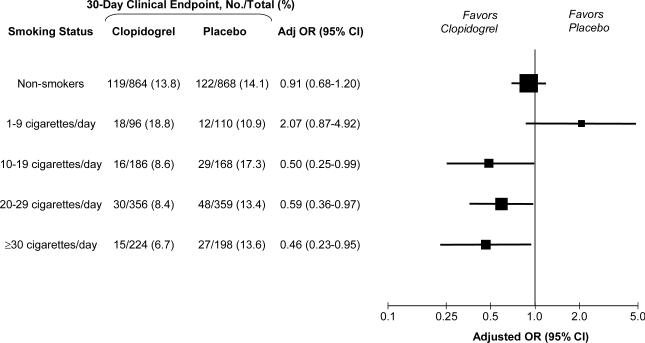
The effect of clopidogrel on the risk of cardiovascular death, myocardial infarction, or recurrent ischemia leading to the need for urgent revascularization by 30 days stratified by intensity of smoking is shown in Figure 2. Overall in the trial, clopidogrel reduced the odds of the 30-day clinical endpoint by 20% (OR 0.80, 95% CI 0.65-0.97, P=0.03). Analogous to the angiographic findings, we observed that the magnitude of treatment benefit with clopidogrel was associated with the degree of smoking. Among non-smokers or those who smoked less than half a pack per day, the addition of clopidogrel had no impact on the rate of the composite clinical (13.7% vs. 14.3%), with an adjusted OR of 0.98 (95% CI 0.75-1.28; P=0.87). In contrast, among those who smoked half a pack a day or more, the addition of clopidogrel reduced the rate of the composite clinical endpoint from 14.3% to 8.0%, with an adjusted OR of 0.54 (95% CI 0.38-0.76; P=0.0004). A test for interaction between smoking, clopidogrel treatment, and the composite clinical endpoint was significant (P=0.006), indicating a significantly greater benefit of clopidogrel in those who smoked at least half a pack per day. Similarly, treatment with clopidogrel significantly reduced the risk of cardiovascular death or MI among those who smoked half a pack a day or more (adjusted OR 0.57, 95% CI 0.38-0.85, P=0.006), whereas it had no effect among non-smokers or those who smoked less than half a pack per day (adjusted OR 0.97, 95% CI 0.71-1.32; P=0.84) (Pinteraction=0.032).
Figure 2. Benefit of Clopidogrel on 30-Day Clinical Endpoint by Smoking Status.
The 30-day clinical endpoint was a composite of death from cardiovascular causes, recurrent myocardial infarction, or recurrent ischemia leading to the need for urgent revascularization. Odds ratios (OR) are adjusted for age, sex, region, hypertension, diabetes, infarct location, time to fibrinolytic therapy, and type of fibrinolytic. For each subgroup, the size of the box is proportional to the number of individuals included in the analysis. The horizontal lines represent the 95 percent confidence intervals (CI).
Rates of TIMI major or minor bleeding were low (<2%) in the entire study. There was no statistically significant interaction between smoking and clopidogrel on the risk of TIMI major or minor bleeding (Pinteraction=0.90).
Discussion
In a randomized, placebo-controlled trial of clopidogrel in over 3400 patients with STEMI, we found that cigarette smoking, which induces one of the key CYP450 enzymes that transforms clopidogrel into its active metabolite, was associated with the magnitude of benefit from clopidogrel on angiographic and clinical outcomes. While clopidogrel had significant benefit in the entire trial cohort, treatment with clopidogrel had approximately twice the efficacy in reducing both the risk of a closed infarct-related artery or death or MI before angiography and the risk of cardiovascular death, recurrent MI, or urgent revascularization over 30 days in patients who smoked at least half a pack of cigarettes per day. This study provides another possible link in the emerging understanding of clopidogrel pharmacology. In exploring clopidogrel resistance or hypo-responsiveness, investigators have now focused on metabolism of clopidogrel to its active metabolite as the key factor. Building on the recent finding that cigarette smoking is an inducer of clopidogrel metabolism leading to higher degrees of platelet inhibition with clopidogrel (12), we now show that cigarette smoking, in turn, is associated with differences in the clinical effectiveness of clopidogrel.
The emerging recognition that factors influencing the metabolism of clopidogrel can impact its pharmacologic and now clinical efficacy is supported by multiple pharmacologic observations. First, CYP1A2 is the predominant enzyme responsible for the first oxidative step in the conversion of clopidogrel to its active metabolite (8). Accelerating the first step would help prevent the pro-drug from being shunted down an esterase-mediated pathway that leads to pharmacologically inactive metabolites. Second, cigarette smoke in general and its polycyclic aromatic hydrocarbons in particular, are known to induce CYP1A2 (10). A study comparing those who smoked half a pack per day or more with non-smokers found a 66% increase in CYP1A2 activity among smokers (14). Third, among heavy smokers, the half-life for dissipation of CYP1A2 induction upon discontinuation of smoking has been estimated to be 38.6 hours, which would easily cover the time frame from symptom onset to clopidogrel loading in our study (15). Fourth, several studies examining the association between platelet aggregation and clinical outcomes have noted trends towards a higher proportion of smokers among patients with less platelet aggregation in response to clopidogrel (4,16,17). Finally, in a recent study of smoking and platelet aggregation by Bliden and colleagues, smokers were noted to have increased platelet inhibition and lower aggregation in response to clopidogrel than non-smokers (12).
Our results suggest a threshold of smoking at least half a pack per day to significantly impact the efficacy of clopidogrel. This threshold was also seen in the study by Bliden and colleagues. However, given the relatively wide confidence intervals around the point estimates in those smoking 10-19, 20-29, and ≥30 cigarettes/day, we cannot definitely exclude a dose-response effect within this range. Nonetheless, the threshold effect now seen in 2 analyses has important implications for interpretation of other studies examining the presence of an interaction between clopidogrel and smoking. Specifically, analyses comparing smokers and non-smokers which include predominantly light smokers may fail to show a significant interaction.
It also is possible that smoking is associated with the magnitude of clinical benefit of clopidogrel for reasons in addition to or other than altering clopidogrel biotransformation into an active metabolite. There are data that smoking is associated with platelet activation in vivo and ex vivo (18-21). Thus if they had more active platelets, smokers might stand to gain greater benefit from more intensive antiplatelet therapy. However, we should note that we did not see any significant difference in angiographic or clinical events in the placebo arm across smoking categories.
Potential limitations of this study merit consideration. First, this was a post-hoc analysis of a completed clinical trial. However, as we have noted above, there are multiple pharmacologic studies that formed the basis for our hypothesis. Second, due to the sample handling and assay complexity necessary for platelet aggregation studies, these evaluations could not be implemented in CLARITY-TIMI 28, a large, multinational clinical trial. Thus, although our findings are consistent with data from recent platelet aggregation studies, within our dataset the mechanistic link between smoking and the efficacy of clopidogrel remains speculative. Third, the subgroup of those who smoked 1-9 cigarettes per day was relatively small; hence the confidence intervals surrounding the effect estimates for clopidogrel in this group were wide. Fourth, smoking status was, of course, not randomized. For that reason, we did not examine the association between smoking status and outcomes, but rather the effect of randomly allocated clopidogrel therapy on outcomes within smoking subgroups. Although the effect modification of smoking on clopidogrel efficacy persisted after careful multivariable adjustment, we cannot exclude residual confounding due to unmeasured or unknown variables. Fifth, we did not adjust for medications that have been associated with altered clopidogrel pharmacology such as statins metabolized by CYP3A4 or proton pump inhibitors (22,23). Use of proton pump inhibitors was not collected on the case report form. Use of statins was collected, but administration of CYP3A4-metabolized statins was not imbalanced across smoking categories. Furthermore, data from 3 randomized trials of clopidogrel (including ours, data not shown) have shown no clinical effect modification by statin therapy (24,25).
In conclusion, cigarette smoking appears to positively modify the beneficial effect of clopidogrel on both angiographic and clinical outcomes. These data highlight the contribution of environmental factors to the inter-patient variability in response to clopidogrel.
Acknowledgments
FUNDING: CLARITY-TIMI 28 was funded by Bristol-Myers Squibb.
DISCLOSURES: The TIMI Study Group reports receiving significant research grant support from AstraZeneca, Bayer Healthcare, Beckman Coulter, Biosite, Bristol-Myers Squibb, CV Therapeutics, Eli Lilly, Genentech, GlaxoSmithKline, Integrated Therapeutics Group, Johnson & Johnson, Merck, Nanosphere, Novartis, Pfizer, Roche Diagnostics, Sanofi-Aventis, Siemens Medical Solutions, Singulex, and Schering-Plough.
Dr. Cannon receives research grant support from: Accumetrics, AstraZeneca, Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Glaxo Smith Kline, Merck, Merck/Schering Plough Partnership, and holds equity in Automedics Medical Systems.
Dr. Sabatine reports receiving research grant support from Sanofi-Aventis and Schering-Plough, and honoraria from Bristol-Myers Squibb and Sanofi-Aventis.
ABBREVIATIONS
- ACS
acute coronary syndrome
- ADP
adenosine diphosphate
- CLARITY-TIMI 28
Clopidogrel as Adjunctive Reperfusion Therapy - Thrombolysis in Myocardial Infarction 28
- CYP
cytochrome P450
- STEMI
ST-segment elevation myocardial infarction
- TFG
TIMI flow grade
- TIMI
Thrombolysis in Myocardial Infarction
References
- 1.The Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 2.Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–89. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 3.Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–13. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 4.Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–5. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 5.Hochholzer W, Trenk D, Bestehorn HP, et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol. 2006;48:1742–50. doi: 10.1016/j.jacc.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 6.Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005;46:1820–6. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 7.Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol. 2005;46:1827–32. doi: 10.1016/j.jacc.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 8.Savi P, Combalbert J, Gaich C, et al. The antiaggregating activity of clopidogrel is due to a metabolic activation by the hepatic cytochrome P450-1A. Thrombosis & Haemostasis. 1994;72:313–7. [PubMed] [Google Scholar]
- 9.O'Donoghue M, Wiviott SD. Clopidogrel response variability and future therapies: clopidogrel: does one size fit all? Circulation. 2006;114:e600–6. doi: 10.1161/CIRCULATIONAHA.106.643171. [DOI] [PubMed] [Google Scholar]
- 10.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–16. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 11.Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36:425–38. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bliden KP, Dichiara J, Lawal L, et al. The association of cigarette smoking with enhanced platelet inhibition by clopidogrel. J Am Coll Cardiol. 2008;52:531–3. doi: 10.1016/j.jacc.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, McCabe CH, Gibson CM, Cannon CP. Design and rationale of Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY) - Thrombolysis in Myocardial Infarction (TIMI) 28 trial. American Heart Journal. 2005;149:227–33. doi: 10.1016/j.ahj.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Vistisen K, Loft S, Poulsen HE. Cytochrome P450 IA2 activity in man measured by caffeine metabolism: effect of smoking, broccoli and exercise. Adv Exp Med Biol. 1991;283:407–11. doi: 10.1007/978-1-4684-5877-0_55. [DOI] [PubMed] [Google Scholar]
- 15.Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76:178–84. doi: 10.1016/j.clpt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Geisler T, Langer H, Wydymus M, et al. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur Heart J. 2006;27:2420–5. doi: 10.1093/eurheartj/ehl275. [DOI] [PubMed] [Google Scholar]
- 17.Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008;29:992–1000. doi: 10.1093/eurheartj/ehn046. [DOI] [PubMed] [Google Scholar]
- 18.Levine PH. An acute effect of cigarette smoking on platelet function. A possible link between smoking and arterial thrombosis. Circulation. 1973;48:619–23. doi: 10.1161/01.cir.48.3.619. [DOI] [PubMed] [Google Scholar]
- 19.Nowak J, Murray JJ, Oates JA, FitzGerald GA. Biochemical evidence of a chronic abnormality in platelet and vascular function in healthy individuals who smoke cigarettes. Circulation. 1987;76:6–14. doi: 10.1161/01.cir.76.1.6. [DOI] [PubMed] [Google Scholar]
- 20.Hung J, Lam JY, Lacoste L, Letchacovski G. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation. 1995;92:2432–6. doi: 10.1161/01.cir.92.9.2432. [DOI] [PubMed] [Google Scholar]
- 21.Harding SA, Sarma J, Josephs DH, et al. Upregulation of the CD40/CD40 ligand dyad and platelet-monocyte aggregation in cigarette smokers. Circulation. 2004;109:1926–9. doi: 10.1161/01.CIR.0000127128.52679.E4. [DOI] [PubMed] [Google Scholar]
- 22.Lau WC, Waskell LA, Watkins PB, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation. 2003;107:32–7. doi: 10.1161/01.cir.0000047060.60595.cc. [DOI] [PubMed] [Google Scholar]
- 23.Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256–60. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 24.Saw J, Steinhubl SR, Berger PB, et al. Lack of adverse clopidogrel-atorvastatin clinical interaction from secondary analysis of a randomized, placebo-controlled clopidogrel trial. Circulation. 2003;108:921–4. doi: 10.1161/01.CIR.0000088780.57432.43. [DOI] [PubMed] [Google Scholar]
- 25.Saw J, Brennan DM, Steinhubl SR, et al. Lack of evidence of a clopidogrel-statin interaction in the CHARISMA trial. J Am Coll Cardiol. 2007;50:291–5. doi: 10.1016/j.jacc.2007.01.097. [DOI] [PubMed] [Google Scholar]