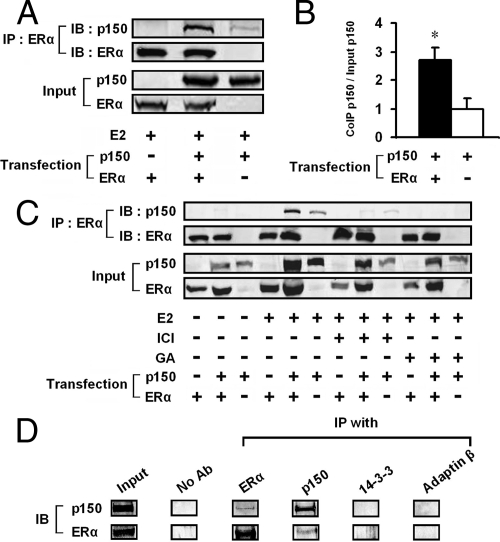
Figure 2.
p150/glued binds to ERα in mammalian cells. A, HEK 293 cells were transiently transfected with plasmids encoding ERα and/or p150/glued. ERα antibodies were used to immunopurify ERα-containing complexes in cells treated with estrogen. Immunoprecipitated proteins were blotted and probed for p150/glued to detect molecular association (top panel). Lysate volumes were adjusted to normalize for equal protein content in each lane. We used a rabbit antiserum [no. EM49 (52)] which recognized transfected rat p150/glued, but not endogenous human protein. A small background band that migrated with p150/glued was consistently seen without transfection of ERα. However, there was a very consistent increase in the precipitated p150/glued found when ERα was included in the precipitates. B, Quantitation of seven coimmunoprecipitations indicated a 2.7-fold increase in precipitated p150/glued in the presence of transfected p150/glued and ERα (with estrogen; compared with control lysates without ERα). *, P < 0.05 for immunoprecipitation with transfected ERα compared with without transfected ERα. C, Complex formation was dependent on estrogen (E2; 10 nm) and inhibited by ICI 182,780 (ICI; 100 nm) and geldanomycin (GA; 1 μm). HEK 293 cells were transfected with cDNAs shown; lysates were analyzed as in panel A. D, p150 and ERα interact in MCF-7 cells. Coimmunoprecipitation was performed in cells treated with DTBP (5 mm in PBS). Buffer or antibodies against ERα, p150, 14-3-3, or adaptin-β were used to pull down protein complexes. Only p150 and ERα antibodies pulled down complexes containing both p150 and ERα. IB, Immunoblotting; IP, immunoprecipitation.