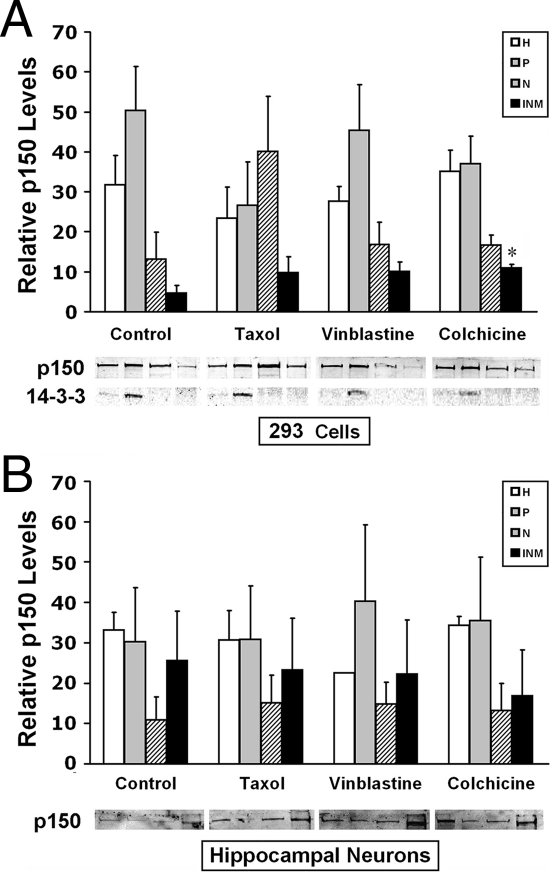
Figure 5.
p150/glued localizes to the nucleus. To assess the subcellular localization of p150/glued, HEK 293 cells (A) and mouse hippocampal neurons (B) were fractionated, and proteins were analyzed by immunoblotting. Fractions were loaded as follows: whole-cell homogenate (H), postnuclear fraction (P), soluble nuclear extract (N), and nuclear matrix fractions (INM). Localization was determined in cells exposed for 6 h to tubulin depolymerizers, colchicine (C; 10 μm), vinblastine (V; 300 nm), or the microtubule stabilizer taxol (T; 500 nm). Colchicine caused a modest increase in nuclear matrix p150/glued only in HEK 293 cells; other treatments did not cause significant changes in p150/glued distribution. 14-3-3 protein was examined to confirm that nuclear fractions were free of cytoplasmic components. Graphs show quantitation of bands in three experiments. Equal volumes of proteins were loaded for each subcellular fraction; thus, quantitation is displayed in relative units of band intensity (y-axis); only comparisons between treatments can be made; there is, in fact, much less nuclear p150/glued compared with cytoplasmic protein; however, nuclear bands appear strong because of increased nuclear protein concentration after centrifugation of organelles. Representative bands from a single experiment are shown below graphs. Statistical analyses were performed to compare groups relative to cells without drug treatment; *, P < 0.05.