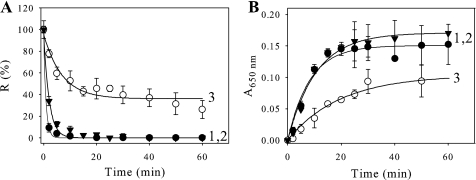
FIGURE 4.
Effect of CP12 mutants on the thermal inactivation and heat-induced aggregation of GAPDH. GAPDH inactivation (A) or aggregation (B) was induced at 43 °C in 30 mm Tris, 100 mm NaCl, 2 mm EDTA, pH 7.9. GAPDH (0.8 μm) was used alone (curve 1, filled circle) or with a 1.6 μm concentration of either C66S mutant (curve 2, filled triangle) or C23S mutant (curve 3). A, the enzyme activity, R, is given as a percentage of the value at zero time. The continuous lines correspond to the best fits to Equation 2, and the parameters are as follows: curve 1, p1 = 100 ± 2%, p2 = 0, and t½ = 0.59 ± 0.04 min; curve 2, p1 = 99 ± 3%, p2 = 0, and t½ = 1.4 ± 0.1 min; curve 3, p1 = 60 ± 6%, p2 = 36 ± 3%, and t½ = 4.8 ± 1.2 min. B, aggregation was followed by measuring absorbance at 650 nm (A650 nm). The continuous lines correspond to the best fits to Equation 1. The parameters are as follows: curve 1, p1 = 0.151 ± 0.006, t½ = 5.7 ± 0.8 min; curve 2, p1 = 0.17 ± 0.01, t½ = 7.4 ± 0.9 min; curve 3, p1 = 0.104 ± 0.005, t½ = 13.6 ± 1.6 min. All data points are means ± S.D. of five experiments for GAPDH alone and of three experiments for GAPDH plus mutant CP12 proteins.