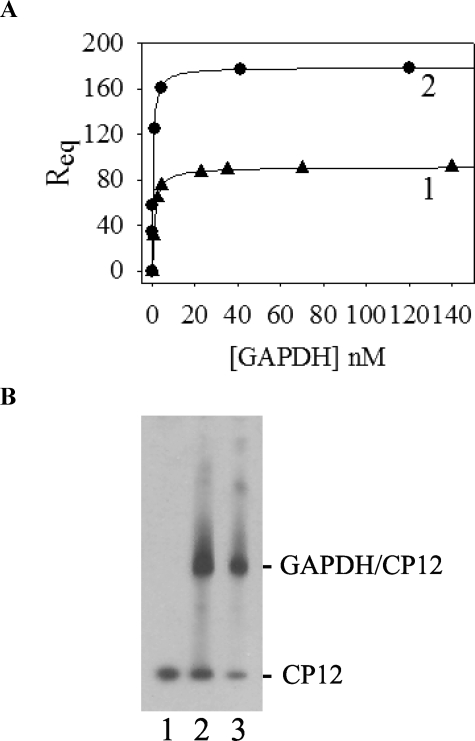
FIGURE 5.
Interaction between GAPDH and reduced CP12. A, surface plasmon resonance binding experiments. The response at equilibrium Req (in RU) was reported as a function of the analyte concentration ([GAPDH]). The experimental points were fitted to a hyperbola, Equation 3. Reduced CP12 (117 RU) was immobilized, and GAPDH was used as analyte (curve 1). Oxidized CP12 (210 RU) was immobilized, and GAPDH was used as analyte (curve 2). B, Western blot analysis of the in vitro reconstitution of GAPDH·CP12 complex. GAPDH and CP12 were mixed in a molar ratio of 1:1. After 1 h at 30 °C, proteins were separated on a native 4–15% gradient gel, transferred to a nitrocellulose membrane, and revealed with antibodies raised against CP12 (1:10,000). Lane 1, CP12 alone (0.03 nmol); lane 2, reconstitution mixture of oxidized CP12 (0.03 nmol) and GAPDH (0.03 nmol); lane 3, reconstitution mixture of reduced CP12 (0.03 nmol) and GAPDH (0.03 nmol). 33 ng of CP12 was loaded in each lane. No band was revealed with recombinant GAPDH alone.