FIGURE 7.
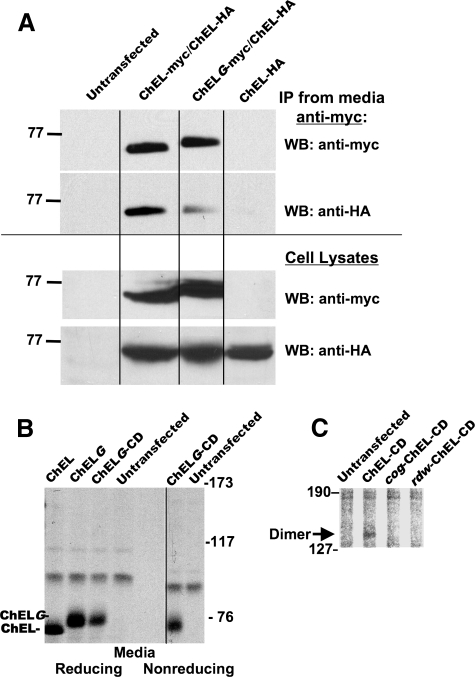
A-A-V to N-A-T mutagenesis to create an N-linked glycosylation site within the α-7/8 helical sequence (Fig. 2B) perturbs subunit contact (35). A, 293 cells were either untransfected or transiently transfected to express either secretory ChEL-Myc or ChEL-Myc bearing the extra glycosylation site (ChELG-Myc), in conjunction with an equal amount of plasmid DNA encoding secretory ChEL-HA. Cells expressing secretory ChEL-HA alone were included as a negative control. Secretion was collected for 24 h. The media were either immunoprecipitated (IP) with anti-Myc before SDS-PAGE (upper two panels) or analyzed directly without immunoprecipitation (lower two panels). Samples underwent Western blotting (WB) with either anti-Myc (to demonstrate recovery of ChEL-Myc or ChELG-Myc) or anti-HA (to examine the extent of co-precipitation of the dimerization partner). Introduction of an N-glycan slowed the electrophoretic mobility of the ChELG-Myc band and decreased co-precipitation of ChEL-HA by 72% (in three such experiments, co-precipitation decreased 52 ± 18%). B, the G mutation was introduced into secretory ChELG-CD. The listed constructs were transiently expressed in 293 cells; secretory ChEL and secretory ChELG (lacking potential for intersubunit covalent bonding) were included as controls. Cells were metabolically labeled and chased for 4 h, and the media were immunoprecipitated with anti-Tg. The samples were analyzed by SDS 5.5%-PAGE under nonreducing (and reducing) conditions as indicated, with no covalent ChELG-CD dimer detected. C, covalent homodimer synthesized in cells expressing ChEL-CD but not in cells expressing cog-ChEL-CD or rdw-ChEL-CD.