FIGURE 2.
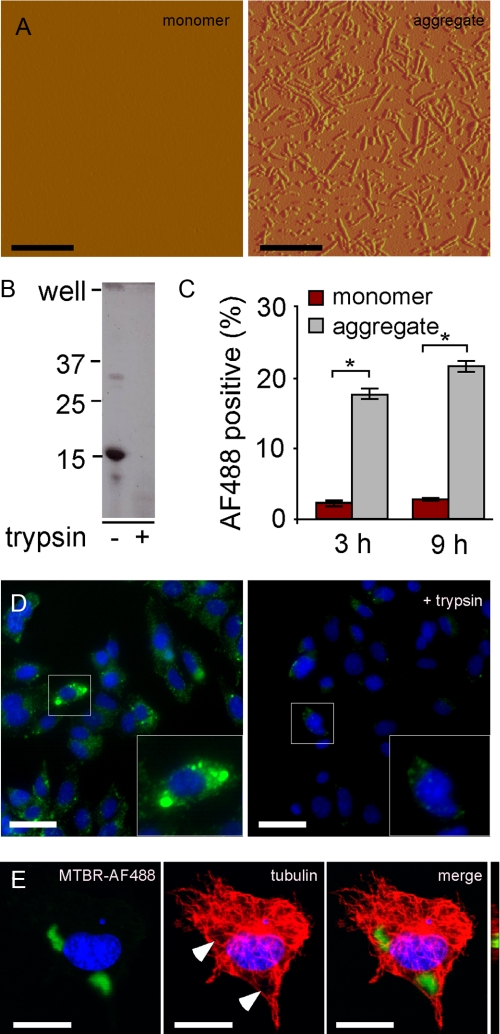
C17.2 cells take up aggregated Tau. A, recombinant MTBR Tau was prepared in vitro and induced to fibrillize using arachidonic acid. Tau monomer is not detectable via AFM. After 24 h of incubation with arachidonic acid, however, Tau is highly aggregated, forming many oligomeric and fibrillar species. Scale bars, 600 nm. B, aggregated Tau was treated with buffer or 0.125% trypsin for 1 min and resolved by SDS-PAGE 4–15% gradient gel, followed by Coomassie stain. Aggregated Tau is very sensitive to trypsin digestion. C, C17.2 cells were exposed to AF488-containing buffer, AF488-labeled monomer, or aggregates. After 3 and 9 h, cells were harvested by 0.25% trypsin treatment. Intracellular AF488 fluorescence was then quantified by flow cytometry. After 3 h, 2.0% of monomer-treated cells scored positive, versus 18% for aggregate-treated cells. After 9 h, 3.0% of monomer-treated cells scored positive, versus 22% for aggregate-treated cells. *, p < 10-6 (unpaired t test, n = 4, 10,000 cells counted per experiment). D, MTBR-AF488 aggregate-treated C17.2 cells with or without trypsin treatment and visualized by confocal microscopy. Scale bar, 30 μm. E, MTBR-AF488 aggregate-treated C17.2 cell stained for tubulin and visualized via confocal microscopy after treatment with 0.25% trypsin illustrates internalized aggregates. Arrows indicate displacement of tubulin. An apical-to-distal slice (bar) obtained from a three-dimensional image rendered from Z-stacks shows MTBR-AF488 in the same plane as tubulin. Scale bar, 10 μm.