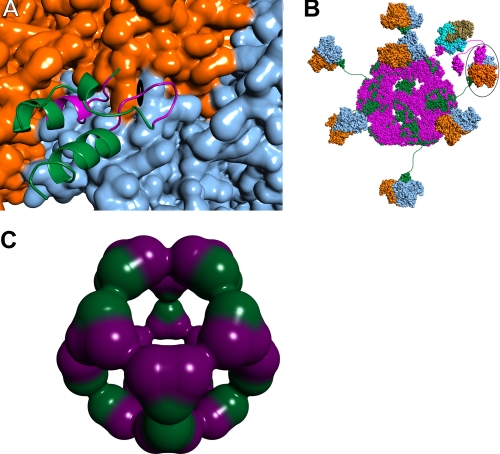
FIGURE 8.
Protein-protein interactions in the native human PDC. A, close-up view of E3BD (ribbons representation) bound to E3 (surface) (14). One monomer of E3 is colored orange, and the other is blue. The approximate position of the dyad axis of the E3 dimer is shown by the black symbol. Most of E3BD is colored green, but those residues with atoms that would clash with a second bound E3BD are shown in purple. B, schematic model of the native human PDC. The dodecahedral 60-meric core of the human PDC is modeled using the structure of the catalytic domain of B. stearothermophilus E2 (44). The E2p polypeptides are colored magenta, with E3BP polypeptides colored green. The E3 dimers are shown in blue and orange, with a single E3BD bound per dimer of E3 (14), as indicated by the data. In this model, it is possible for 20 E3 dimers to bind; only 7 are shown for clarity. A single E1p heterotetramer docked to the E1pBD of E2p is shown, with the α subunits shown in tan and the β subunits in cyan. The structure of the human versions of E1p bound to E1pBD is unknown; shown here is the structure from B. stearothermophilus (45). The circled E3 has an LBD of E. coli E2p docked to the active site. E2p and E3BD are therefore noncovalently cross-linked via their mutual interaction with E3. C, possible arrangement of E2p and E3BP components in a 40/20 core. Shown is a dodecahedral arrangement of 20 heterotrimers composed of 2 E2p proteins (purple) and one E3BP (green).