Abstract
The recall and re-experiencing of a personal emotional event (emotional imagery) are thought to evoke neural activity in the central nervous system that can affect the physiology of bodily states. It has been proposed that the more active the neural systems previously engaged in the emotional experience, and the more active the bodily state associated with that experience, the more vivid the emotional imagery is. The sympathetic nervous system (SNS) and the gastrointestinal system (GI) are engaged in emotional reactions. On this basis, we hypothesized that vivid emotional imagery would be accompanied by strong increases in gastrointestinal and sympathetic nervous system activity. To test this hypothesis, 17 healthy participants performed emotional imagery of strong autobiographical memories involving various emotional states (happy, fear, disgust, sadness, anger). SNS and GI changes, measured by skin conductance and electrogastrogram, respectively, correlated positively with subjective ratings of arousal during the imagery. However, the SNS changes did not correlate with ratings of emotional imagery vividness, and even more intriguingly, the GI changes correlated strongly and negatively with vividness ratings. To account for these findings, we propose that in highly vivid imagery experience, the central nervous system is simulating the whole emotional experience strongly, and bodily information plays a lesser role. In low vivid imagery experience, the central nervous system is not simulating very strongly the emotional experience, and information coming from the body (including the GI system) plays a greater role. This interpretation is set forth in the context of Damasio's (1999) theoretical framework, which predicts such a dissociation between a “body loop” and an “as if body loop” for the experiencing and re-experiencing of emotions and feelings.
Keywords: emotion, imagery, gastrointestinal, gut, EGG, arousal, body, vividness, skin conductance
1. Introduction
The Oxford English dictionary defines imagination as the act of forming mental images of something not present to the senses or never before wholly perceived in reality (www.oed.com). Imagination can influence the way we see, behave, and remember. Furthermore, it is an essential part of the creative process, and most notably, it has been proposed to have an important role in psychopathologies, such as phobias, depression, body dysmorphic syndrome, psychosis, and anxiety disorders (Holmes and Hackmann, 2004, Pratt et al., 2004). Vivid emotional images are one of the main features of post-traumatic stress disorder (PTSD). In fact, one of the cognitive therapies suggested for PTSD is the manipulation of these images to reduce their emotional potency (Andrade et al., 1997, Cook et al., 1988, Cuthbert and Lang, 1989, Cuthbert et al., 2003).
To recall and re-create emotional images from imagination, it is thought that neural activity related to the emotional experience is re-evoked (Lang, 1979). On this view, the ability for people to recall an emotional event is related to the ability to re-experience various sensory modalities, such as smells, sights, sounds, and physiological responses. For instance, the smell of a fearful object can trigger an emotional memory, because the olfactory system is activated, and can reactivate the other sensory modalities involved in previous encounters with that object. This activity in turn influences the physiology of bodily states, such as increases in skin conductance and heart rate, while one is imagining the experience of encountering a fearful object (Kosslyn et al., 2001, Lang et al., 1993, Sharot et al., 2004). That is, the more the brain and the body re-enact the situation, the better and more vivid is the image. Therefore, psychophysiological responses should reflect the vividness of the image.
Emotional imagery studies that have tried to correlate psychophysiological data with vividness of imagery have shown inconsistent findings. Several studies have demonstrated that there is a positive correlation between overall imagery scores and psychophysiological responses (such as skin conductance) during imagery (Lang et al., 1980, Miller et al., 1987, Cook et al., 1988, Suler, 1985, Van Diest et al., 2001). However, Laor and colleagues (1999) observed that overall image vividness scores (measured by a version Bett's Mental Imagery Questionnaire—QMI; Sheehan, 1967) correlated negatively with psychophysiological responses (heart rate variability) when PTSD patients were presented with trauma-related stimuli. Furthermore, no correlation between imagining and physiological variables was seen in healthy comparison participants in Laor et al.'s study. Also, Cook and colleagues (1988) found that overall image vividness correlated with skin conductance increase only in patients with specific phobias, but not in patients with general agoraphobias or social anxiety.
This mixed picture of the relationship between imagery and psychophysiology response may be due to the fact that our understanding of the bodily changes in an emotional situation is far from complete. This is likely due at least in part to the Herculean task of capturing the entire set of physiological changes during emotional reactions. Recently, there have been findings of association between specific emotional experiences and specific patterns of autonomic change (Kreibig et al., 2007, Levenson, 2003, Levenson et al., 1992, Rainville et al., 2006), suggesting that the bodily response to emotion is complex and does not reflect a single parameter, such as “arousal.” However, these studies have tended to focus on the cardiac, respiratory and sudomotor systems, which are relatively easy to access from a technical perspective. Thus, there is much in the landscape of the bodily response to emotion that remains unexplored.
A system that has been almost completely neglected in emotion research is the gastrointestinal tract (GI). However, this picture is starting to change, and the interest in the role of the GI in regulating behaviors has been increasing. For instance, Vianna and Tranel (2006) have shown that the peak amplitude of the electrogastrogram (EGG), a measure of gastric myoelectric activity, correlates with the subjective ratings of arousal in normal participants while watching emotional video clips. A study of patients with irritable bowel syndrome found that hypnotically induced anger and excitement were associated with increases in colonic motility, heart rate, and respiration rate (Whorwell et al., 1992). Also, Blomhoff and colleagues (2000) showed changes in rectal tone associated with emotionally charged words. While these results show an effect of an emotional state on the GI state, changes in the GI state can also have an effect on emotional state. Patients with Crohn's disease (an inflammatory bowel syndrome) who do not have any psychiatric comorbidity have higher subjective ratings of arousal while watching short film clips compared to healthy participants (Vianna et al., 2006). Crohn's disease patients present symptoms of altered gut motility and behavior, and inflammation of the lining of digestive tract. Therefore, aberrant gastrointestinal signals may lead to a higher subjective feeling of arousal. This demonstrates how crucial the GI system is in emotional states that are triggered by external stimuli. The question remains as to what role, if any, the GI system plays in emotional states that are triggered by mental imagery.
It has been proposed that greater activation of the autonomic nervous system (ANS) is associated with more vivid emotional imagery (Lang, 1979, Lang and Cuthbert, 1984). According to Lang's theory of emotional imagery (Lang, 1979), to have a strong and vivid recall of an emotional event, one must reactivate all the systems previously involved in the emotional process, and this includes parasympathetic and sympathetic reactivation. Therefore, we hypothesized that the vividness of emotional imagery would be correlated with the activity of the gastrointestinal tract, as measured by the EGG. Because it is believed that highly arousing emotion is correlated with highly vivid emotional imagery (Buchanan, 2007; Talarico et al., 2004), we also measured skin conductance, given that this measure is a well established index of sympathetic activation (Bradley, 2000, Burch and Greiner, 1960, Edelberg, 1972). We predicted that skin conductance and subjective ratings of arousal would correlate positively with the vividness of emotional imagery.
2. Methods
2.1. Subjects
Seventeen healthy volunteers (8 male; 9 female) were recruited from the University of Iowa Hospitals and Clinics and from among the University of Iowa students and staff. All procedures were approved by the Internal Review Board of the Human Subjects Office of the University of Iowa College of Medicine.
2.2. Experimental procedure
Participants engaged in an emotional imagery task that has been described previously (Damasio et al., 2000, Rainville et al., 2006). Prior to the experimental day, participants described personal emotional episodes in as much detail as possible. Upon arrival to the psychophysiological laboratory, participants gave informed consent, and the physiological monitoring equipment was installed. Then, the physiological recording started and the participant was instructed to close his/her eyes and relive the target emotion as vividly as possible while remembering the autobiographical episode, and to try to maximize the target emotion until the experimenter asked them to stop. The experimenter provided verbal cues to guide mental evocation based on the participant's prior description of the events and emphasized the events that precipitated the target emotion. Participants indicated by a slight movement of the hand when they started to feel the emotion or when they were in the imagined neutral scenario. Representative emotional experiences included such examples as the following: (a) neutral: getting ready that morning, the last time they did the laundry; (b) happy: being accepted in graduate school, watching a son graduating from college; (c) fear: being followed in a dark alley, almost having a car accident; (d) disgust: having to use an extremely dirty bathroom, having to clean dog excrement with worms; (e) sadness: the death of a close relative, girlfriend leaving to another town; (f) anger: the process of divorce, insurance problems after car accident. The experimenter then stopped further cueing and the physiological recording was time-stamped for off-line analysis. Participants were told that they could stop experiencing the emotion after 120 s and the physiological recording was time-stamped again and the data saved. Different experimental conditions were separated by at least five minutes during which the participants were invited to relax. Each participant completed all emotion conditions (anger, fear, happiness, sadness, disgust) and the control neutral condition. The order of emotion conditions was counterbalanced across participants. One participant had excessive movement during the experimental paradigm, and the EGG data had to be discarded, leaving a total of 16 participants with usable EGG recordings.
2.3. Subjective ratings of the emotional experience
After each trial, participants provided Likert-scale ratings (1-7) of the arousal, valence, and vividness of the target emotion felt during the trial. For emotional arousal, participants rated the intensity of their emotional experience with 1 corresponding to no emotion, and 7 to extremely intense emotion. For emotional valence, the participants rated the pleasantness, with 1 corresponding to extremely unpleasant, 7 to extremely pleasant, and 4 to neutral. Participants also reported the primary emotion felt during each trial, and were given a choice of happiness, sadness, anger, fear or disgust. For vividness, the participants rated how vivid was the imagery, with 1 being not vivid, and 7 extremely vivid.
2.4. Physiological measures
Physiological activity was monitored continuously using Biopac amplifiers (Biopac Systems Inc.) and the data were digitized (200 Hz), recorded and processed using the MP100 system and AcqKnowledge software (Biopac Systems Inc.). EGG was recorded using a standard 3 leads montage (Einthoven lead 2 configuration). In addition to those target physiological channels, we recorded palmar skin conductance (SC) of both hands. SC was recorded using disposable Ag-AgCl skin electrodes. Electrodes were placed on the thenar and hypothenar eminences of the right hand. Skin conductance output voltage was amplified by a factor of 5 μS/V and low-pass filtered at 10 Hz.
2.5. Physiological signal processing
All physiological recordings were continuously monitored during the experiment and visually inspected off-line. Recording artifacts were identified and corrected by interpolation or else discarded.
2.5.1. EGG
The EGG is a reliable, noninvasive method of recording gastric myoelectrical activity (Muth et al., 1998). It is recorded by placing electrodes on the surface of the skin over the antrum of the stomach. The frequencies recorded by the EGG are identical to the frequencies of electrical activity when recorded in or directly on the stomach and the frequency of stomach contractions when they occur. As contractile activity in the stomach increases, the amplitude of the normal 3-cpm EGG rhythm increases (Stern et al., 2000).
EGG data obtained during each emotional imagery condition were subjected to fast Fourier transform (FFT) with a Hamming window. Maximum spectral value was calculated for each condition. Because of significant between-subject variability in the measure, the values for each participant were transformed into z-scores. Spectral estimates were summed for the following bands: normogastria (2.5-3.5 cpm), tachygastria (3.75-9.75 cpm), bradygastria (0.5-2.5 cpm), and total power (0.5-11 cpm). Normogastria is associated with normal rhythm of the stomach of a healthy individual. The function of bradygastria and tachygastria are not well understood. It is known that tachygastria activity is usually associated with nausea (Stern et al., 2000). Because of the length of emotional imagery, bradygastria analysis was not included in the EGG, as the FFT analysis would have required longer data acquisition set-up.
2.5.2. Skin conductance
Skin conductance activity was measured as the area under the curve over a given time interval (Damasio et al., 2000). Raw skin conductance data were low-pass filtered to remove high frequency noise. The slow downward drift in baseline skin conductance level was removed using a moving difference function with a difference interval of 0.05 s (10 points for a 200 Hz sampling rate). Because the imagery had slightly different time lengths, the area under the curve was divided by total film clip time. For statistical analysis, z-scores of the participants were calculated.
2.6. Data analysis
Correlations between arousal and psychophysiology measures, between valence and psychophysiology measures, and between vividness and psychophysiological measures were performed according to procedures outlined by Lang and colleagues (1993). The psychophysiological measures were ordered according to the subjective response for each subject. Afterwards, the psychophysiological measures were averaged at each subjective response across participants. This was followed by the correlation analysis between subjective responses and averaged physiological measures.
3. Results
3.1. Correlations of psychophysiological measures and self-report of emotions
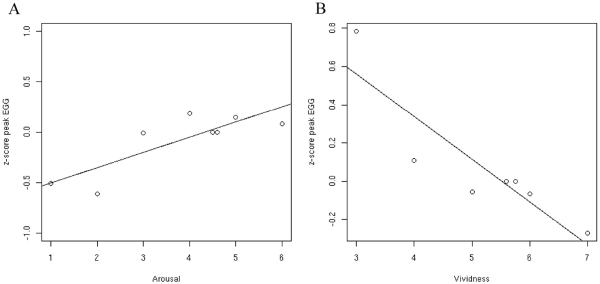
The main question of the study is whether there would be a strong correlation between the psychophysiology measures and vividness of emotional imagery. There was a strong positive correlation between the maximum spectral z-score values of the EGG and subjective ratings of arousal (r=0.83, P=0.01; Figure 1A). This result confirms a previous report that the EGG is a sensitive index of subjective arousal (Vianna and Tranel, 2006). However, contrary to our prediction, there was a strong but negative correlation between the maximum spectral z-score values of the EGG and vividness ratings (r=-0.75, P=0.04; Figure 1B). The correlation between EGG and valence was small and not significant (r=-0.2; P=0.504).
Figure 1.
Correlation of psychophysiological measures and subjective ratings. The data analysis was conducted according to a method described previously (Lang et al., 1993). Data points reflect mean psychophysiological response for a level of arousal or vividness. A. z-score peak amplitude of EGG correlates with subjective ratings of arousal (r=0.8325, P=0.01); B. z-score of peak amplitude EGG correlates negatively with ratings of how vivid was the imagery (r=-0.75, P=0.04).
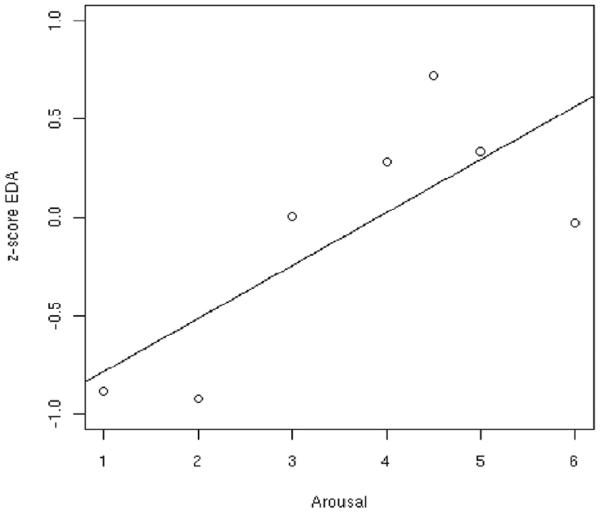
There was a strong positive correlation between skin conductance z-scores and subjective ratings of arousal (r=0.76, P=0.047; Figure 2). The correlations with valence (r=-0.30; P=0.47) and vividness (r=-0.31; P= 0.49) ratings were smaller and not statistically significant. The latter finding—i.e., the non-significant correlation between skin conductance and vividness—is not consistent with our prediction.
Figure 2.
Corrleation of skin conductance and arousal. The data analysis was conducted according to a method described previously (Lang et al., 1993). z-score of skin conductance correlates with subjective ratings of arousal (r=0.76, P=0.047);
We also calculated the correlation between vividness and arousal. Contrary to our expectations, the correlation between vividness and arousal ratings was small and not statistically significant (r=0.30, P=0.43). Also, the correlation of vividness and valence ratings was almost zero (r=0.05, P=0.59).
4. Discussion
In this study, we hypothesized that electrogastrogram activity (EGG) and skin conductance (SC) would correlate with vividness of emotional imagery. Contrary to our hypothesis, vividness ratings did not correlate with skin conductance activity. Also, vividness ratings were strongly but negatively correlated with changes in electrogastrogram activity.
SC is considered a direct measure of sympathetic nervous system activation, and it is also well established that SC is correlated with subjective ratings of arousal (Bradley, 2000, Burch and Greiner, 1960, Edelberg, 1972). According to emotional imagery theory, an increase in sympathetic nervous system activity should increase the vividness of the imagery (Lang, 1979). While some studies have shown a correlation between skin conductance and vividness scores (Lang et al., 1980, Miller et al., 1987), other studies have not produced such supporting evidence (Cook et al., 1988, Laor et al., 1999). Laor and colleagues (1999) have suggested that the lack of correlation between ratings of vividness and psychophysiological activity is due to the experimental setting. In fact, Bywalters and colleagues (2004a) have shown that vividness and arousal ratings are correlated in delayed recall of emotional pictures. However, when performing a similar experience with emotional imagery, neither arousal nor skin conductance activity correlated with vividness ratings (Bywalters et al., 2004b). The authors suggest that the relationship between arousal and vividness changes when the imagery is dependent on long-term memory. The lack of correlation between arousal and vividness or skin conductance activity and vividness observed in our study is in agreement with Bywalters and colleagues' data and interpretation.
In order to interpret our results, it is critical to understand what the EGG actually measures. EGG is a reliable tool for measuring the myoelectrical activity of the stomach (Smout et al., 1980, Stern et al., 2000). It is important to note that there are two sources of the myoelectrical activity in stomach that can be measured by EGG, the gastric slow waves and spike potentials. The gastric slow wave is an ever-present periodic activity produced by the interstitial cells of Cajal (ICC). ICC serves as a stomach-pacemaker, and its activity does not necessarily indicate stomach contractions. Spike potentials are time-locked with the slow wave activity, and are directly associated with stomach contractions (Chen et al., 1995). Gastric contractions are always captured by the EGG, but changes in the electrogastrogram wave does not mean necessarily change in stomach contractibility (Chen et al., 1999, Huizinga, 2001). Instead of being a direct measure of gastric contraction, one can refer to EGG as a measure of the “gastric state” with two components: ICC's activity and the smooth muscle contractions.
Even though the SNS has a strong modulatory effect on gut activity, the latter is not directly controlled by the SNS. The SNS does not directly innervate the gut musculature as seen, for example, in the heart (Hansen, 2003). Instead, the SNS sends projections to the enteric nervous system that controls the state of gastric activity. Most importantly, the magnitude of the inhibitory effect on gut motility by the SNS is dependent on the ongoing gut and enteric nervous system activity. Also, increased SNS activity can also increase the gut sensitivity and perception of gut distension (De Ponti et al., 1996, Iovino et al., 1995). Therefore, one might argue that the observed increase in sympathetic activity, as measured by SC, is inhibiting the stomach motility and increasing its sensitivity. The increase of SC might reflect a general measure of arousal, whereas the change in EGG would reflect the stomach state (increased sympathetic activity would lead to a decreased motility and increased sensitivity) and would be a more sensitive measure than SC for correlating with vividness ratings.
Thus, a mixed picture arises: Increased SC reflects an increase in sympathetic nervous system activity. Increased sympathetic nervous system activity has two consequences on gastric activity: 1) increased sensitivity of GI (rendering EGG a more sensitive tool for correlating with vividness ratings); and 2) decreased gastric motility (inferred by the negative correlation of EGG and vividness ratings). However, unrelated to the effects of the sympathetic nervous system on the GI, there is a strong and reliable positive correlation of EGG and ratings of arousal. This positive correlation has been shown previously and was replicated by the present data (Vianna and Tranel, 2006, Vianna et al., 2006).
The seemingly contradictory results can be explained by a general theory of how body signals are important for feelings and decision-making (Damasio, 1994, 1999). This theory is an extension of the James-Lange theory of emotion. Originally, the James-Lange theory stated that the way a person feels is dependent on bodily changes (Damasio, 1994, 2001; James, 1884; Lange, 1887; Prinz, 2006). Damasio has gone further to propose that body state changes can be simulated by the brain without changes in bodily states actually happening. That is, the brain constructs a predictive model of the changes that can occur in the body. The simulation of body states is called the “as-if body loop.” For the as-if body loop, it is purported that an emotional stimulus activates brain areas responsible for mapping visceral states. This activation bypasses the body proper, but for the brain systems involved in emotion, feeling, and decision making, it is “as if” the body is changing. This leads to a faster response without having to wait for a slow physiological change. The as-if body loop would be advantageous whenever there is a need for an anticipated body response and it could occur in moments of a highly vivid internal simulation, such as vivid emotional imagery. To have vivid emotional imagery, at least under the conditions utilized in our study, participants may actively shut out and shut down signals from “outside,” including ones from outside the brain, and hence use more exclusively the as-if body loop.
The inverse correlation of the gastrointestinal activity, as measured by the EGG, and the subjective ratings of vividness, can be explained by the as-if body loop. Whenever, the experience is highly vivid, participants may rely more on neural structures than on body responses; hence, there is lower amplitude of the gastrointestinal tract in relation to the vivid ratings. On the other hand, when the central nervous system is not able to recall vividly the emotional experience, the overall feeling of emotion relies more on the body signals, and less on the as-if loop. The as-if body loop also agrees with Lang's theory of emotional imagery, as all neural systems are active during a vivid emotional imagery, but the neural systems are vivid emotional imagery.
It is worthy of note that Pham and colleagues (Pham et al., 2001) have shown that people who are “non-vivid imagers” are more influenced in the decision making process by images than people who are vivid imagers. According to the authors vivid imagers rely more on non-salient information than non-vivid imagers for the decision making process. We can hypothesize from our data that people who are vivid imagers do not rely so much on “gut feelings,” because they have all the information needed (from the as-if body loop), whereas the non-vivid imagers rely on imagery cues to make decisions. In the light of the growing body of evidence that decision-making relies on body cues (Bechara et al., 1997, Bechara et al., 2005), and the different imagining situations, one can ask if there are differential roles for the body, and most importantly for the gastrointestinal tract, in different types of decision-making, feelings, and imagination. We envision that there will be an affirmative answer to this question, but these issues warrant further research.
Acknowledgments
Supported by NINDS P01 NS19632 and NIDA R01 DA022549
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ANDRADE J, KAVANAGH D, BADDELEY A. Eye-movements and visual imagery: a working memory approach to the treatment of post-traumatic stress disorder. Br J Clin Psychol. 1997;36(Pt 2):209–23. doi: 10.1111/j.2044-8260.1997.tb01408.x. [DOI] [PubMed] [Google Scholar]
- BECHARA A, DAMASIO H, TRANEL D, DAMASIO AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- BECHARA A, DAMASIO H, TRANEL D, DAMASIO AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9:159–62. doi: 10.1016/j.tics.2005.02.002. discussion 162-4. [DOI] [PubMed] [Google Scholar]
- BLOMHOFF S, SPETALEN S, JACOBSEN MB, VATN M, MALT UF. Intestinal reactivity to words with emotional content and brain information processing in irritable bowel syndrome. Dig Dis Sci. 2000;45:1160–5. doi: 10.1023/a:1005502119461. [DOI] [PubMed] [Google Scholar]
- BRADLEY MM. Measuring emotion: Behavior, feeling, and physiology. In: LANE R, NADEL L, editors. Cognitive Neuroscience of Emotion. Oxford University Press; New York: 2000. [Google Scholar]
- BUCHANAN TW. Retrieval of emotional memories. Psychol Bull. 2007;133:761–79. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURCH NR, GREINER TH. A bioelectric scale of human alertness: concurrent recordings of the EEG and GSR. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:183–93. [PubMed] [Google Scholar]
- BYWALTERS M, ANDRADE J, TURPIN G. Determinants of the vividness of visual imagery: The effects of delayed recall, stimulus affect and individual differences. Memory. 2004a;12:479–488. doi: 10.1080/09658210444000160. [DOI] [PubMed] [Google Scholar]
- BYWALTERS M, ANDRADE J, TURPIN G. Intrusive and non-intrusive memories in a non-clinical sample: The effects of mood and affect on imagery vividness. Memory. 2004b;12:467–478. doi: 10.1080/09658210444000089. [DOI] [PubMed] [Google Scholar]
- CHEN JD, LIN Z, WU Q, MCCALLUM RW. Non-invasive identification of gastric contractions from surface electrogastrogram using back-propagation neural networks. Med Eng Phys. 1995;17:219–25. doi: 10.1016/1350-4533(95)95713-k. [DOI] [PubMed] [Google Scholar]
- CHEN JD, ZOU X, LIN X, OUYANG S, LIANG J. Detection of gastric slow wave propagation from the cutaneous electrogastrogram. Am J Physiol. 1999;277:G424–30. doi: 10.1152/ajpgi.1999.277.2.G424. [DOI] [PubMed] [Google Scholar]
- COOK EW, 3RD, MELAMED BG, CUTHBERT BN, MCNEIL DW, LANG PJ. Emotional imagery and the differential diagnosis of anxiety. J Consult Clin Psychol. 1988;56:734–40. doi: 10.1037//0022-006x.56.5.734. [DOI] [PubMed] [Google Scholar]
- CUTHBERT BN, LANG PJ. Imagery, memory, emotion: a psychophysiological analysis of clinical anxiety. In: TURPIN G, editor. Handbook of clinical psychophysiology. Wiley; Chichester, UK: 1989. [Google Scholar]
- CUTHBERT BN, LANG PJ, STRAUSS C, DROBES D, PATRICK CJ, BRADLEY MM. The psychophysiology of anxiety disorder: fear memory imagery. Psychophysiology. 2003;40:407–22. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- DAMASIO A. The feeling of what happens: body and emotion in the making of consciousness. Harcourt; Orlando, Fl: 1999. [Google Scholar]
- DAMASIO A. Fundamental feelings. Nature. 2001;413:781. doi: 10.1038/35101669. [DOI] [PubMed] [Google Scholar]
- DAMASIO AR. Descartes' Error. Putnam; New York: 1994. [Google Scholar]
- DAMASIO AR, GRABOWSKI TJ, BECHARA A, DAMASIO H, PONTO LL, PARVIZI J, HICHWA RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- DE PONTI F, GIARONI C, COSENTINO M, LECCHINI S, FRIGO G. Adrenergic mechanisms in the control of gastrointestinal motility: from basic science to clinical applications. Pharmacol Ther. 1996;69:59–78. doi: 10.1016/0163-7258(95)02031-4. [DOI] [PubMed] [Google Scholar]
- EDELBERG R. Electrical activity of the skin: its measurements and uses in psychophysiology. In: GREENFIELD NS, STERNBACH RA, editors. Handbook of Psychophysiology. Holt, Rinehart and Winston; New York: 1972. [Google Scholar]
- HANSEN MB. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52:1–30. [PubMed] [Google Scholar]
- HOLMES EA, HACKMANN A. A healthy imagination? Editorial for the special issue of memory: mental imagery and memory in psychopathology. Memory. 2004;12:387–8. doi: 10.1080/09658210444000124. [DOI] [PubMed] [Google Scholar]
- HUIZINGA JD. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. II. Gastric motility: lessons from mutant mice on slow waves and innervation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1129–34. doi: 10.1152/ajpgi.2001.281.5.G1129. [DOI] [PubMed] [Google Scholar]
- IOVINO P, AZPIROZ F, DOMINGO E, MALAGELADA JR. The sympathetic nervous system modulates perception and reflex responses to gut distention in humans. Gastroenterology. 1995;108:680–6. doi: 10.1016/0016-5085(95)90439-5. [DOI] [PubMed] [Google Scholar]
- JAMES W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- KOSSLYN SM, GANIS G, THOMPSON WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2:635–42. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- KREIBIG SD, WILHELM FH, ROTH WT, GROSS JJ. Cardiovascular, electrodermal, and respiratory response patterns to fear- and sadness-inducing films. Psychophysiology. 2007;44:787–806. doi: 10.1111/j.1469-8986.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- LANG PJ. Presidential address, 1978. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- LANG PJ, CUTHBERT BN. Affective information processing and the assessment of anxiety. J Behav Assess. 1984;6:369–95. doi: 10.1007/BF01321326. [DOI] [PubMed] [Google Scholar]
- LANG PJ, GREENWALK MK, BRADLEY MM, HAMM AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- LANG PJ, KOZAK MJ, MILLER GA, LEVIN DN, MCLEAN A., JR. Emotional imagery: conceptual structure and pattern of somato-visceral response. Psychophysiology. 1980;17:179–92. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- LANGE CG. Über Gemuthsbewegungen. T. Thomas; Leipzig: 1887. [Google Scholar]
- LAOR N, WOLMER L, WIENER Z, SHARON O, WEIZMAN R, TOREN P, RON S. Image vividness as a psychophysiological regulator in Posttraumatic Stress Disorder. J Clin Exp Neuropsychol. 1999;21:39–48. doi: 10.1076/jcen.21.1.39.946. [DOI] [PubMed] [Google Scholar]
- LEVENSON RW. Blood, sweat, and fears: the autonomic architecture of emotion. Ann N Y Acad Sci. 2003;1000:348–66. doi: 10.1196/annals.1280.016. [DOI] [PubMed] [Google Scholar]
- LEVENSON RW, EKMAN P, HEIDER K, FRIESEN WV. Emotion and autonomic nervous system activity in the Minangkabau of west Sumatra. J Pers Soc Psychol. 1992;62:972–88. doi: 10.1037//0022-3514.62.6.972. [DOI] [PubMed] [Google Scholar]
- MILLER GA, LEVIN D, KOZAK MJ, COOK EW, 3RD, MCLEAN A, JR., LANG PJ. Individual differences in imagery and the psychophysiology of emotion. Cognition and Emotion. 1987;1:367–390. [Google Scholar]
- MUTH ER, THAYER JF, STERN RM, FRIEDMAN BH, DRAKE C. The effect of autonomic nervous system activity on gastric myoelectrical activity: does the spectral reserve hypothesis hold for the stomach? Biol Psychol. 1998;47:265–78. doi: 10.1016/s0301-0511(97)00030-6. [DOI] [PubMed] [Google Scholar]
- PHAM MT, MEYVIS T, ZHOU R. Beyond the obvious: chronic vividness of imagery and the use of information in decision making. Organizational Behavior and Human Decision Process. 2001;84:226–253. doi: 10.1006/obhd.2000.2924. [DOI] [PubMed] [Google Scholar]
- PRATT D, COOPER M, HAKMANN A. Imagery and its chracteristics in people who are anxious about spiders. Behavioural and Cognitive Psychotherapy. 2004;32:165–176. [Google Scholar]
- PRINZ JJ. Gut Reactions: A Perceptual Theory of Emotion. Oxford University Press; New York: 2006. [Google Scholar]
- RAINVILLE P, BECHARA A, NAQVI N, DAMASIO AR. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int J Psychophysiol. 2006;61:5–18. doi: 10.1016/j.ijpsycho.2005.10.024. [DOI] [PubMed] [Google Scholar]
- SHAROT T, DELGADO MR, PHELPS EA. How emotion enhances the feeling of remembering. Nat Neurosci. 2004;7:1376–80. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- SHEEHAN PW. A shortened form of Betts' questionnaire upon mental imagery. J Clin Psychol. 1967;23:386–9. doi: 10.1002/1097-4679(196707)23:3<386::aid-jclp2270230328>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- SMOUT AJ, VAN DER SCHEE EJ, GRASHUIS JL. What is measured in electrogastrography? Dig Dis Sci. 1980;25:179–87. doi: 10.1007/BF01308136. [DOI] [PubMed] [Google Scholar]
- STERN RM, KOCH KL, MUTH ER. The gastrointestinal system. In: CACCIOPO JT, TASSINARY LG, BERNSTON GG, editors. Handbook of Psychophysiology. 2nd ed. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- SULER JR. Imagery ability and the experience of affect by free associative imagery. Journal of Mental Imagery. 1985;9:101–110. [Google Scholar]
- TALARICO JM, LABAR KS, RUBIN DC. Emotional intensity predicts autobiographical memory experience. Mem Cognit. 2004;32:1118–32. doi: 10.3758/bf03196886. [DOI] [PubMed] [Google Scholar]
- VAN DIEST I, WINTERS W, DEVRIESE S, VERCAMST E, HAN JN, VAN DE WOESTIJNE KP, VAN DEN BERGH O. Hyperventilation beyond fight/flight: respiratory responses during emotional imagery. Psychophysiology. 2001;38:961–8. doi: 10.1111/1469-8986.3860961. [DOI] [PubMed] [Google Scholar]
- VIANNA EPM, TRANEL D. Gastric myoelectrical activity as an index of emotional arousal. Int J Psychophysiol. 2006;61:70–6. doi: 10.1016/j.ijpsycho.2005.10.019. [DOI] [PubMed] [Google Scholar]
- VIANNA EPM, WEINSTOCK J, ELLIOT D, SUMMERS R, TRANEL D. Increased feelings with increased body signals. SCAN. 2006;1:56–62. doi: 10.1093/scan/nsl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHORWELL PJ, HOUGHTON LA, TAYLOR EE, MAXTON DG. Physiological effects of emotion: assessment via hypnosis. Lancet. 1992;340:69–72. doi: 10.1016/0140-6736(92)90394-i. [DOI] [PubMed] [Google Scholar]