Abstract
Metabolic syndrome (MetS) is associated with increased incidence of diabetes and cardiovascular disease (CVD). Prospective clinical trials with alpha-tocopherol (AT) have not yielded positive results. Because AT supplementation decreases circulating gamma-tocopherol (GT), we evaluated supplementation with GT (800 mg/day), AT (800 mg/day), the combination or placebo for 6 weeks alone AT and GT concentrations, biomarkers of oxidative stress, and inflammation in subjects with MetS (n=20/group). Plasma AT and GT levels increased following supplementation with AT alone or GT alone or in combination. AT supplementation significantly decreased GT levels. Urinary alpha- and gamma-CEHC, metabolites of the respective Ts, also increased correspondingly, i.e., alpha-CEHC with ATand gamma-CEHC with GT supplementation, compared to placebo. HsCRP levels significantly decreased in the combined AT+GT group. LPS-activated whole blood release of IL-1 and IL-6 did not change. There was a significant decrease in TNF with AT alone or in combination with GT. Plasma MDA/HNE and lipid peroxides were significantly decreased with AT, GT, or in combination. Nitrotyrosine levels were significantly decreased only with GT or GT+AT but not with AT compared to placebo. Thus, the combination of AT and GT supplementation appears to be superior to either supplementation alone on biomarkers of oxidative stress and inflammation and needs to be tested in prospective clinical trials to elucidate its utility in CVD prevention.
Keywords: Antioxidant, Tocopherol, Inflammation, Oxidative stress, Nitrative stress
Introduction
Metabolic syndrome affects 1 in 4 adults in the United States and is associated with increased incidence of diabetes and cardiovascular disease [1,2]. Several lines of evidence support a role for oxidative stress and inflammation in atherogenesis. In supplementation studies in humans, alpha-tocopherol (AT), the major form of vitamin E, has been shown to significantly decrease biomarkers of oxidative stress and inflammation [3,4]. The results of the majority of prospective vitamin E clinical trials, however, have been disappointing, as reviewed previously [5]. All of the studies discussed were carried out with AT supplements. AT decreases plasma gamma-tocopherol concentrations (GT), as a result of the function of the hepatic AT transfer protein, which preferentially incorporates AT into the plasma [6], as well as increasing GT metabolism. This observation has often been suggested as an explanation for the null results observed with AT supplementation in the majority of prospective clinical trials, especially since GT concentrations are inversely associated with increased morbidity and mortality due to cardiovascular disease [7–10]. Studies in animal models show that GT is a potent an-tioxidant and is effective in significantly decreasing protein nitration [7–10].
Despite the promises of GT as an effective antioxidant and anti-inflammatory agent in vitro, with regard to GT supplementation in humans, the literature is scanty. In a clinical trial, Himmelfarb et al. [11] enrolled 15 uremic patients undergoing dialysis. Five patients were supplemented with RRR-AT (300 mg/day) and 10 received mixture of tocopherols (60% RRR-γT, 28% RRR-δT, and 18% RRR-AT) for a duration of 14 days. Tocopherol administration increased serum CEHC concentrations in both healthy subjects and hemodialysis patients. Administration of the GT-enriched supplement, but not AT, to hemodialysis patients significantly reduced levels of the prototypic marker of inflammation, hsCRP. Liu et al. [12] supplemented healthy subjects with placebo, all-rac AT(100 mg/day), or mixed tocopherols (comprising 100 mg GT, 20 mg δT, and 20 mg AT) for 8 weeks. Mixed tocopherols but not AT supplementation decreased ADP-induced platelet aggregation. Both AT and mixed tocopherol supplementation resulted in reduced PKC and increased SOD and NO release. Recently, Wu et al. [13], in a double-blind, placebo-controlled trial, in 55 patients with type 2 diabetes who were randomly assigned to receive AT (500 mg/day), mixed tocopherols, or placebo for 6 weeks showed that both AT- and GT-enriched supplementation resulted in reduced plasma F2-isoprostanes. These studies point to an important role for either GT or combined AT+GT supplementation on biomarkers of oxidative stress and inflammation and CVD; however, the relevance of these studies is unclear since most of the studies discussed used either mixed tocopherol preparations or GT-enriched supplements rather than GT alone.
Thus, in this study, we tested the effect of GT supplementation alone and in combination with AT on biomarkers of oxidative stress and inflammation in subjects with MetS. Our hypothesis was that GT would be a more effective anti-inflammatory agent than AT.
Subjects and methods
Subjects
The study was approved by the Institutional Review Board at UC Davis and all subjects gave informed consent. Participants were recruited from the community through advertisements in local newspapers and flyers. Eighty participants who were not currently vitamin or antioxidant users were recruited. Participants were included without restriction to race or socioeconomic status. Participants were recruited who had at least three features of metabolic syndrome, including: (i) waist circumference (men >40 inches; women >35 inches); (ii) tri-glycerides >150 mg/dl; (iii) blood pressure >130/80 mm Hg; (iv) fasting glucose >100 mg/dl; (v) HDL cholesterol <40 mg/dl in men and <50 mg/dl in women.
Exclusion criteria for the study include the following: patients with coronary artery disease; use of lipid-lowering drugs or drugs affecting lipid metabolism; individuals with a BMI> 30 kg/m2; diabetes or hypertension on drug treatment; aspirin therapy; antioxidant supplements; anti-inflammatory drugs; liver, renal, or uncompensated metabolic/hormonal disorders; infection; cancer; recent major surgery or illness; postmenopausal women on estrogen replacement therapy; pregnant women; and children.
Study design
This was a randomized, placebo-controlled double-blind trial. Participants were randomly assigned to receive either AT (RRR) alone (800 mg/day), GT (800 mg/day), the combination (800 mg each/day, AT and GT combo), or placebo for 6 weeks. All subjects were advised to take 2 capsules/day with meals. AT, GT, combo capsules, and respective placebos were provided by Archer Daniel Midland Corp (Decatur, IL).
Fasting blood (50 ml) and a 24-h urine sample were collected at baseline and after 6 weeks for measurement of indices of oxidative stress and inflammation, including whole blood cytokines (IL-1b, TNF-α, IL-6), hsCRP, and urinary nitrotyrosine. In addition, a complete blood count, plasma lipid profile, kidney (creatinine) and liver function (AST, ALT) test, blood glucose test, and TSH were assayed at these time points.
HsCRP levels were measured using a high-sensitivity assay (Beckman LxPro) as described previously [14]. The cytokines IL-1β, IL-6, and tumor necrosis factor (TNF)-α were measured in the supernatant of lipopolysaccharide (100 ng/ml)-activated whole blood after a 24-h incubation at 37°C using a highly sensitive immunoassay (R&D Systems, Minneapolis, MN). The intraassay CV was <4%. Plasma MDA and HNE and lipid peroxides were measured using colorimetric assays as described previously [15]. Nitrotyrosine levels in urine were measured using an ELISA from Oxis International and expressed as micromoles of nitrotyrosine per milligram urinary creatinine. The intraassay CV was <10%.
Measurement of vitamin E and vitamin E metabolites
Plasma AT and GT concentrations were determined by HPLC with electrochemical detection as described [16]. Plasma or urinary CEHCs were extracted following addition of an internal standard (trolox) and enzymatic hydrolysis (1 mg β-glucuronidase, type H-1, Sigma-Aldrich (St. Louis, MO), and then analysis by LC/MS as described [17].
Statistics
Results were analyzed using GraphPadPrizm software. Data are expressed as mean±SD for parametric and median and interquartile range for nonparametric data. Two-way ANOVA was performed and time and treatment interaction effects were assessed followed by post hoc t tests or Mann Whitney and statistical significance was set at P<0.05.
Results
Subject baseline characteristics are provided in Table 1. Subjects in the four groups were matched for age and BMI. There were no significant differences in glucose and lipid profile among the four groups.
Table 1.
Subject characteristics and blood parameters
| Placebo |
AT |
GT |
AT+GT |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | Baseline | Post | Baseline | Post | |
| Age (years) | 56±11 | 51±11 | 50±9 | 57±14 | ||||
| Gender | 6 M/14F | 4 M/16F | 2 M/18F | 7 M/13F | ||||
| BMI(kg/m−2) | 39±15 | 35±5 | 33±7 | 35±7 | ||||
| TC (mg/dl) | 215±41 | 199±49 | 216±28 | 234±44 | 227±36 | 222±42 | 222±54 | 223±53 |
| HDL-C (mg/dl) | 39±8 | 39±10 | 41±10 | 41±11 | 43±9 | 42±7 | 42±8 | 42±10 |
| TG (mg/dl) | 198±124 | 183±101 | 208±133 | 209±112 | 200±102 | 170±82 | 176±83 | 200±40 |
| Glucose (mg/dl) | 106±22 | 103±20 | 118±63 | 112±55 | 99±28 | 102±22 | 121±63 | 112±47 |
Data are expressed as mean±SD.
Plasma AT and GT
Plasma AT levels increased significantly following 6 weeks of supplementation with AT (P<0.001) or with the AT and GT combo (P<0.001, Fig. 1a). The postsupplementation plasma AT levels from either the AT or the AT-GT combo were not statistically different from each other (44±4 μM vs 55±4 μM, respectively).
Fig. 1.
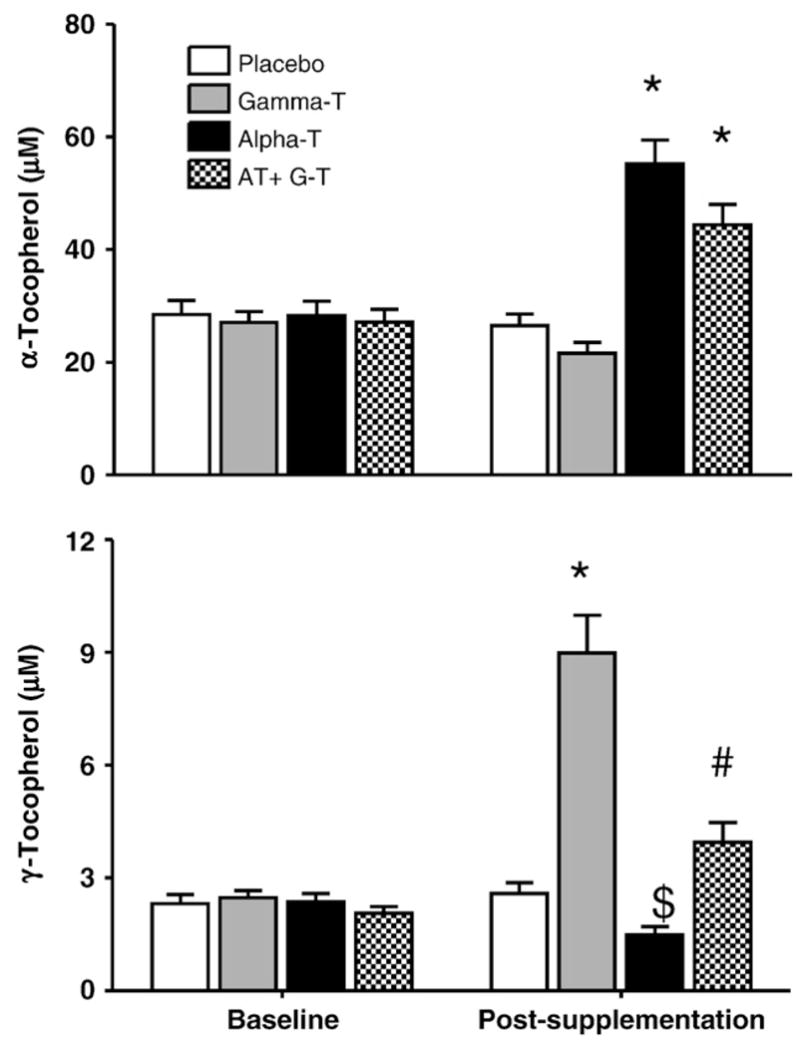
Effect of AT, GT, and AT+GT supplementation on plasma AT and plasma GT Levels: Plasma AT and GT levels were measured at baseline and at Week 6 (postsupplementation) in the four groups as described under Subjects and methods. *P<0.001 compared to baseline and placebo. $P<0.01 compared to baseline and GT. #P<0.05 compared to GT alone.
GT supplementation for 6 weeks doubled plasma GT levels, but did not change plasma AT concentrations significantly. Importantly, the increase in plasma GT was significantly less with the AT-GT combination (P<0.001, AT+GT compared to GT postsupplementation). Furthermore, plasma GT decreased 37% following supplementation with AT (Fig. 1b).
Plasma CEHCs
AT and GT metabolites (A- and G-CEHC, respectively) are markers of vitamin E supplementation and metabolism [18]. AT supplementation increased plasma A-CEHC, while GT supplementation increased plasma G-CEHC (P<0.001, baseline compared to postsupplementation; Fig. 2a and Fig. 2b, respectively). AT+ GT increased both plasma A- and G-CEHCs, respectively, compared with placebo.
Fig. 2.
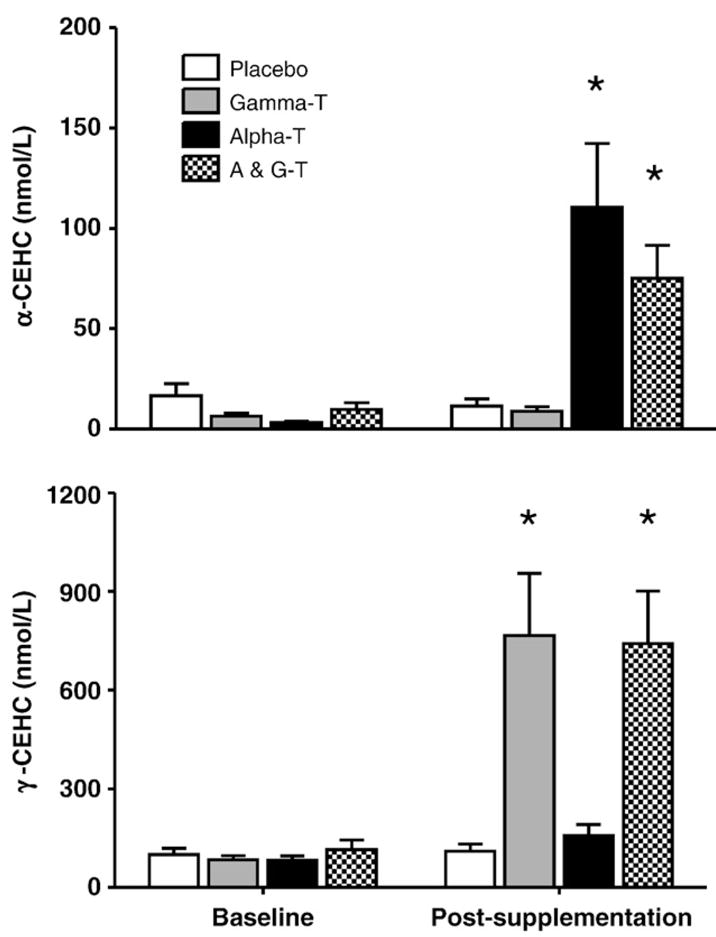
Effect of AT, GT, and AT+GT supplementation on plasma alpha and gamma CEHC levels: Plasma A-CEHC and G-CEHC levels were measured at baseline and at Week 6 (postsupplementation) in the four groups as described under Subjects and methods. *P<0.001 compared to baseline and placebo.
Urinary vitamin E metabolites
As CEHCs are water-soluble excretion products, we measured the urinary CEHC levels. Following AT or AT+GT supplementation, urinary A-CEHC significantly increased (P<0.001; baseline compared to postsupplementation; Fig. 3a). However, post-supplementation urinary A-CEHC following AT alone was significantly greater than following the AT+GT supplement (P<0.001). Urinary G-CEHC increased significantly with both AT+GT or GT alone with no statistically significant differences between the two groups (Fig. 3b).
Fig. 3.
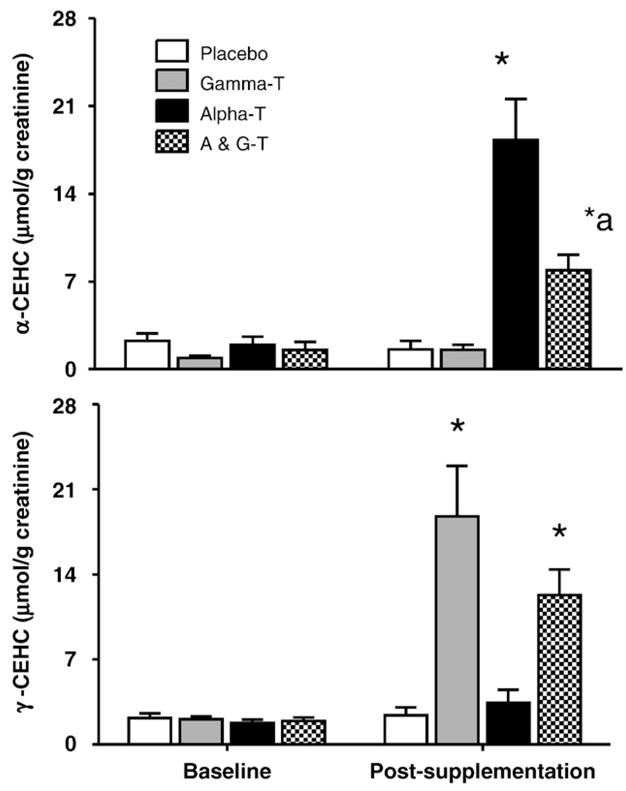
Effect of AT, GT, and AT+GT supplementation on urinary alpha and gamma CEHC levels: Urine A-CEHC and G-CEHC levels were measured at baseline and at Week 6 (postsupplementation) in the four groups as described under Subjects and methods and standardized to creatinine. *P <0.001 compared to baseline and placebo; *aP<0.01 compared to AT.
Markers of inflammation and oxidative stress
HsCRP Levels were significantly decreased following AT, GT, and AT+GT supplementation compared to baseline; however, only the combination resulted in significant differences compared to placebo (median reduction 15%, P<0.01; Fig. 4a). There was no change in whole blood release of IL-1b and IL-6; however, AT therapy alone and in combination with GT significantly decreased whole blood release of TNF-α (Table 2). With regard to biomarkers of oxidative stress, plasma lipid peroxides as well as MDA+HNE significantly decreased with both AT and GT either alone or in combination compared to placebo (P<0.01, Table 2). Furthermore, nitrotyrosine levels in urine were significantly decreased following GT supplementation, either alone or in combination with AT; AT alone failed to decrease urinary nitrotyrosine (P<0.02; Fig. 4b).
Fig. 4.
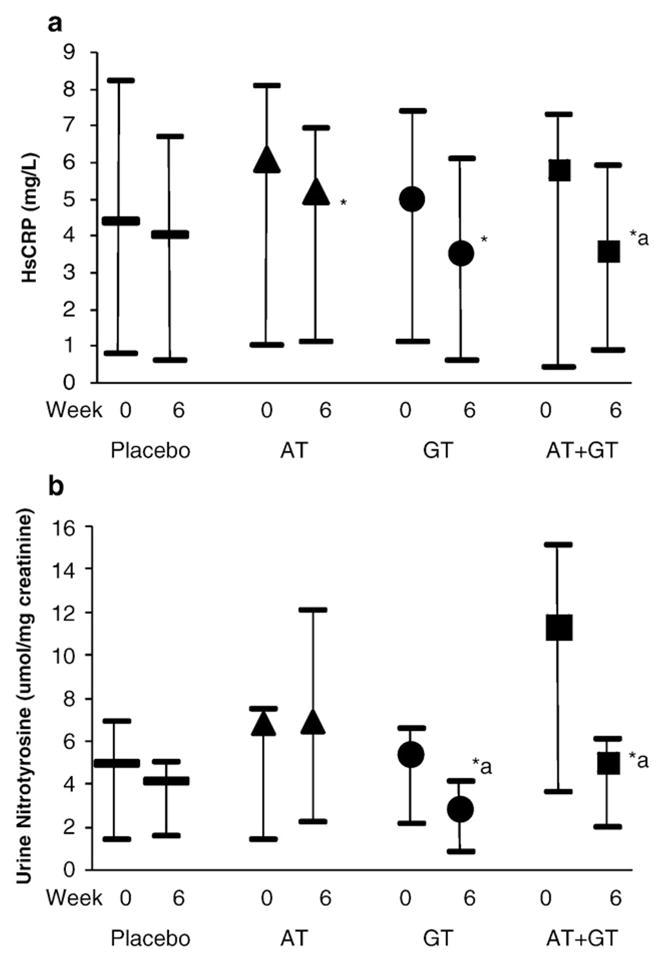
(a) Effect of AT, GT, and AT+GT supplementation on HsCRP Levels: HsCRP levels were measured at baseline and at Week 6 (postsupplementation) in the four groups as described under Subjects and methods. *P<0.001 compared to baseline and *aP<0.02 compared to placebo. (b) Effect of AT, GT, and AT+GT supplementation on urine nitrotyrosine levels: HsCRP levels were measured at baseline and at Week 6 (postsupplementation) in the four groups as described under Subjects and methods and standardized to creatinine. *aP<0.02 compared to baseline and placebo.
Table 2.
Biomarkers of oxidative stress and inflammation
| Placebo |
AT |
GT |
AT+GT |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | Baseline | Post | Baseline | Post | |
| IL-1(ng/L) | 8.3±5.9 | 9.3±7.4 | 7.4±3.8 | 6.2±3.6 | 6.9±3.9 | 6.5±4.1 | 7.9±6.6 | 9.0±6.1 |
| IL-6 (ng/L) | 3.4±2.6 | 3.9±2.6 | 3.1±2.2 | 3.3±2.4 | 3.2±1.7 | 2.9±1.4 | 3.7±2.1 | 4.0±1.8 |
| TNF (ng/L) | 0.31 (0.04, 0.66) | 0.39 (0.21, 0.88) | 0.27 (0.11, 0.59) | 0.23 (0.08, 0.48)* | 0.26 (0.06, 0.67) | 0.24 (0.06, 0.53) | 0.28 (0.06, 0.7) | 0.14 (0.05, 0.41)*a |
| Plasma MDA+ HNE (μM) | 2.5±1.3 | 2.5±1.6 | 2.9±1.4 | 2.0 ±0.9*a | 2.8±1.2 | 1.8 ±0.6*a | 2.9±1.7 | 2.1 ±1.0*a |
| Plasma Lipid Peroxides (μM) | 3.3±1.2 | 3.2±1.2 | 3.5±1.0 | 2.1±1.0*a | 3.4±1.2 | 2.0±0.6*a | 3.5±1.2 | 1.9±0.6*a |
P<0.05 compared to placebo (time and treatment interaction).
P<0.05 compared to baseline (time interaction).
Discussion
MetS confers an increased risk of future diabetes and CVD [1,2]. Several studies have shown that MetS is also associated with increased inflammation and oxidative stress. The NCEP has recommended that therapeutic lifestyle changes are the primary treatment strategy for MetS. Dietary micronutrients with anti-oxidant and anti-inflammatory potential could be alternative strategies to reduce the cardiovascular burden in MetS. AT is a potent antioxidant and anti-inflammatory agent; however, prospective CVD clinical trials have not shown promising results [3,4]. This could in part be explained by the fact that AT supplementation results in decreased levels of GT, the main form of dietary vitamin E. While previous supplementation studies with mixed tocopherols that were enriched with GT have yielded significant reductions in biomarkers of oxidative and nitrative stress, no studies have been conducted with purified GT alone in MetS [7–13]. Thus, in this study, we provide novel data with regard to purified GTsupplementation compared to ATor AT+GT alone on bioavailability of AT and GTas well as on biomarkers of oxidative stress and inflammation in MetS subjects.
In accordance with other studies, we show that plasma AT levels increased significantly with AT supplementation following 8 weeks of supplementation with ATalone or in combination with GT. Furthermore, previous studies have shown that AT supplementation decreases plasma GT levels as observed in our study (37% decrease) [6]. It has been suggested that AT can replace GT in lipid membranes, resulting in decreased plasma GT levels and thus mixed tocopherol supplementation could potentially avoid adverse biological effects. We show here that the decrease in GT levels with AT supplementation is abrogated with the combination of AT and GT. The concentration of tocopherols appears to be saturable, resulting in increased metabolism to CEHCs. In healthy subjects, previously, it has been suggested that urinary CEHC can be used as a biomarker of adequate tocopherol stores [19]. In this study, we have performed comprehensive analyses of both plasma and urinary CEHCs and show that AT supplementation resulted in increased levels A-CEHC and, for the first time, show that with purified GT supplementation, there is increased G-CEHC with no effect on A-CEHC. It is also interesting to note that the combination of AT+GT resulted in significantly increased A- and G-CEHCs; however, the level of A-CEHCs was significantly decreased compared to AT supplementation alone, indicating the importance of the hepatic AT transport protein in regulating the metabolic fate of the tocopherols and the preference for GT metabolism to G-CEHC potentially interfering with AT metabolism.
CRP is a prototypic marker of inflammation and a risk marker for CVD [20]. In addition, several lines of evidence indicate that CRP may be an active participant in atherosclerosis. Also, studies have reported that AT supplementation (≥800 IU/day) results in significant decreases in hsCRP levels [21,22]. However, there is a paucity of data examining tocopherol supplementation in MetS subjects and no data with regard to purified GT supplementation on biomarkers of inflammation in MetS. In this placebo-controlled study, we report that only the combination of AT+ GT resulted in significant reduction in hsCRP compared to placebo and either AT or GT supplementation significantly decreased HsCRP levels compared to baseline but these were not significantly different from those of placebo. In a previous small study, there was a significant reduction in hsCRP in hemodialysis patients following administration of GT-enriched mixed tocopherols (600 mg/day for 14 days) but not AT supplementation alone [11]. Furthermore, the effect of GT supplementation alone was not studied. Recently, Wu et al. [13] reported that neither AT or mixed tocopherol supplementation (500 mg/day for 6 weeks) resulted in any significant changes in CRP, MCP-1, IL-6, or TNF-α levels. In accordance with these studies, we also failed to observe any significant change in whole blood release of cytokines, except TNF-α, which was significantly different with AT alone or AT+GT therapy.
Several studies have demonstrated that AT has antioxidant effects. In this study, we show that GT supplementation alone significantly reduces biomarkers of oxidative stress, plasma MDA and HNE, and lipid peroxides, similar to AT alone or the combination. Previous studies in vitro have shown that GT is also highly potent in quenching reactive nitrogen species compared to AT [23]. Thus, we also examined urinary nitrotyrosine levels following GT supplementation alone and in combination with AT. While AT alone failed to affect urinary nitrotyrosine, GT alone as well as in combination with AT resulted in significant reduction in urinary nitrotyrosine. Previously, Cooney et al. [24] have shown that GT reacts with nitrogen dioxide to produce nitric oxide while AT forms an intermediate analog, such as the quinone in vitro. Also, AT and nitric oxide cooperatively inhibit lipid peroxidation better than AT/ascorbate combination in vitro [25,26]. However, it is important to note that these studies were performed in an vitro system. In the present study, in vivo, it appears that GT is more potent than AT in decreasing nitrosative stress, and this needs to be confirmed in future larger studies, also examining mechanisms for these observations.
Thus, in conclusion, we report for the first time in MetS subjects that the combination of AT + GT therapy results in significant increases in AT and GT concentrations as well as their metabolites in plasma and urine, as well as significant reductions in hsCRP, urinary nitrotyrosine, and lipid peroxides. These results point to the superiority of combined AT + GT supplementation in ameliorating both oxidative and nitrative stress and inflammation in MetS subjects. Future studies will be directed at examining mechanisms for these changes and testing the effect of combined supplementation on cardiovascular events in high risk populations such as chronic kidney disease and metabolic syndrome.
Acknowledgments
We acknowledge grants from NIH K24AT00596, RO1AT00005, CNRU, to M.G.T; DK067930; Office Dietary Supplements. We thank Arpita Basu for help with subject recruitment and technical assistance.
Abbreviations
- AT
cardiovascular disease
- CVD
cardiovascular disease
- GT
gamma-tocopherol
- MetS
metabolic syndrome
- TNF
tumor necrosis factor
References
- 1.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program–Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 3.Jialal I, Devaraj S. Scientific evidence to support a vitamin E and heart disease health claim: research needs. J Nutr. 2005;135:348–353. doi: 10.1093/jn/135.2.348. [DOI] [PubMed] [Google Scholar]
- 4.Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, Johnston CS, Kelly FJ, Kraemer K, Packer L, Parthasarathy S, Sies H, Traber MG. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–745. doi: 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- 5.Devaraj S, Jialal I. Failure of vitamin E in clinical trials: is gamma-tocopherol the answer? Nutr Rev. 2005;63:290–293. doi: 10.1111/j.1753-4887.2005.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 6.Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol levels in humans. J Nutr. 1985;115:807–813. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- 7.Devaraj S, Traber MG. Gamma-tocopherol, the new vitamin E? Am J Clin Nutr. 2003;77:530–531. doi: 10.1093/ajcn/77.3.530. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Q, Christen S, Shigenaga MK, Ames BN. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 10.Saldeen T, Li D, Mehta JL. Differential effects of alpha- and gamma-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis. J Am Coll Cardiol. 1999;34:1208–1215. doi: 10.1016/s0735-1097(99)00333-2. [DOI] [PubMed] [Google Scholar]
- 11.Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, et al. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978–991. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Wallmon A, Olsson-Mortlock C, Wallin R, Saldeen T. Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms. Am J Clin Nutr. 2003;77:700–706. doi: 10.1093/ajcn/77.3.700. [DOI] [PubMed] [Google Scholar]
- 13.Wu JHY, Ward NC, Indrawan AP, et al. Effects of AT and mixed tocopherol supplementaiton on markers of oxidative stress and inflammation in type 2 diabetes. Clin Chem. 2007;53:511–519. doi: 10.1373/clinchem.2006.076992. [DOI] [PubMed] [Google Scholar]
- 14.Devaraj S, Autret BC, Jialal I. Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. Am J Clin Nutr. 2006;84:756–761. doi: 10.1093/ajcn/84.4.756. [DOI] [PubMed] [Google Scholar]
- 15.O’Byrne DJ, Devaraj S, Grundy SM, Jialal I. Comparison of the antioxidant effects of Concord grape juice flavonoids alpha-tocopherol on markers of oxidative stress in healthy adults. Am J Clin Nutr. 2002;76:1367–1674. doi: 10.1093/ajcn/76.6.1367. [DOI] [PubMed] [Google Scholar]
- 16.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols and ubiquinones. J Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 17.Leonard SW, Gumpricht E, Devereaux MW, Sokol RJ, Traber MG. Quantitation of rat liver vitamin E metabolites by LC-MS during high-dose vitamin E administration. J Lipid Res. 2005;46:1068–1075. doi: 10.1194/jlr.D400044-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled α- and γ-tocopherol demonstrate faster plasma γ-tocopherol disappearance and greater γ-metabolite production. Free Radic Biol Med. 2005;38:857–866. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol—an underestimated vitamin? Ann Nutr Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 20.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44:6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- 21.Devaraj S, Jialal I. Alpha tocopherol supplementation decreases serum C-reactive protein and monocyte interleukin-6 levels in normal volunteers and type 2 diabetic patients. Free Radic Biol Med. 2000;29:790–792. doi: 10.1016/s0891-5849(00)00420-2. [DOI] [PubMed] [Google Scholar]
- 22.Jialal I, Devaraj S. Inflammation and atherosclerosis: the value of the high-sensitivity C-reactive protein assay as a risk marker. Am J Clin Pathol. 2001;116:S108–S115. doi: 10.1309/J63V-5LTH-WYFC-VDR5. [DOI] [PubMed] [Google Scholar]
- 23.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha-and gamma-tocopherol. Mol Aspects Med. 2007 doi: 10.1016/j.mam.2007.01.003. [Electronic publication ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. g-Tocopherol detoxification of nitrogen dioxide: superiority of a-tocopherol. Proc Natl Acad Sci U S A. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubbo H, Radi R, Anselmi D, Kirk M, Barnes S, Butler J, Eiserich JP, Freeman BA. Nitric oxide reaction with lipid peroxyl radicals spares alpha tocopherol during lipid peroxidation. Greater oxidant protection from the pair nitric oxide/alpha-tocopherol than alpha-tocopherol/ascorbate. J Biol Chem. 2000;275(15):10812–10818. doi: 10.1074/jbc.275.15.10812. [DOI] [PubMed] [Google Scholar]
- 26.De Groot H, Hegi U, Sies H. Loss of <alpha>0tocopherol upon exposure to nitric oxide or the sydnonimine SIN-1. FEBS Lett. 1993;315(2):139–142. doi: 10.1016/0014-5793(93)81150-x. [DOI] [PubMed] [Google Scholar]


