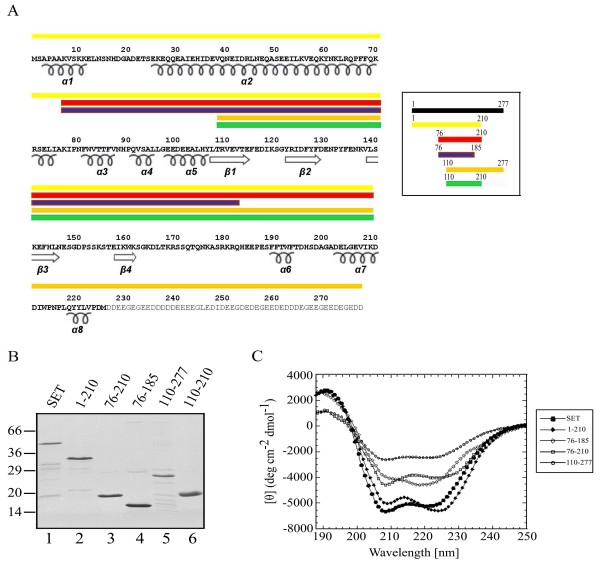
Figure 2.
Design and structural integrity of SET/TAF-Iβ deletion constructs. (A) Aminoacid sequence and secondary structure elements of SET/TAF-1β (PBD code 2E250) and its deletion mutants; SET/TAF-Iβ (1–210) (yellow), SET/TAF-Iβ (76–210) (red), SET/TAF-Iβ (76–185) (blue), SET/TAF-Iβ (110–277) (orange), SET/TAF-Iβ (110–210) (green)(insert). Residues in the acidic stretch are shown in grey. (B) Wild type SET/TAF-Iβ and its deletion mutants were expressed and purified as GST-tagged proteins and subsequently the GST moiety was removed, as indicated in the Methods section. The proteins were analysed by SDS-PAGE and Coomassie blue staining. (C) Circular dichroism spectra of wild type SET/TAF-Iβ (1–277) (filled hexagon), SET/TAF-Iβ (1–210) (filled diamond), SET/TAF-Iβ 110–277 (open hexagon), SET/TAF-Iβ 76–185 (open diamond) and SET/TAF-Iβ (76–210) (open square). Stock solutions of SET/TAF-IB polypeptides were in 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5 mM DTT, 1 mM EDTA in a concentration range of 10–75 μM.