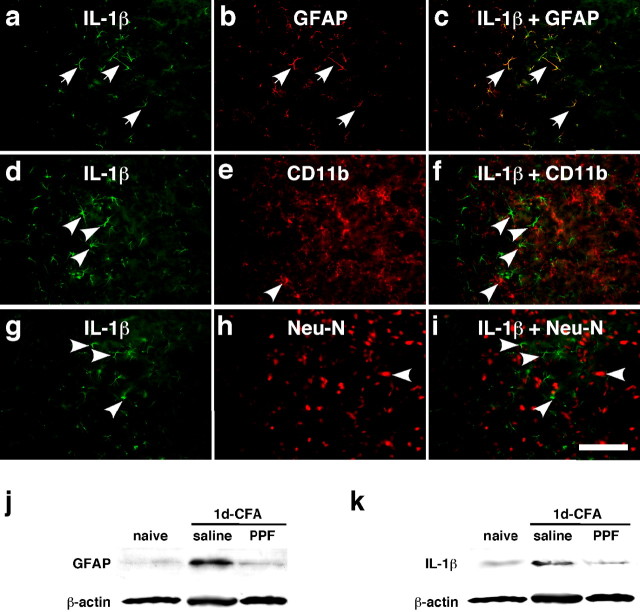
Figure 4.
Colocalization of IL-1β with GFAP and the effect of a glial inhibitor on GFAP and IL-1β upregulation. a–i, Brainstem tissue sections were processed for IL-1β (a, d, g) and double labeled with GFAP (b), CD11b (e), or NeuN (h) 1 d after masseter inflammation. Photomicrograph images are from the ventral portion of the Vi/Vc transition zone. IL-1β immunoreactivity is shown as green fluorescence after reacting with anti-goat IgG conjugated with Cy2. GFAP-, CD11b-, and NeuN-LI are visualized with Cy3 conjugated with anti-rabbit IgG (red). Overlap of a and b reveals double fluorescence (c; yellow/orange), which was only identified for IL-1β and GFAP staining (arrows in a, b, c), indicating the selective induction of IL-1β in astroglia. d–f and g–i show lack of double labeling of IL-1β with CD11b, a marker of microglia, or NeuN, a marker of neurons. The single-labeled neurons in d–i are indicated by arrowheads. Scale bar, 0.1 mm. j, k, Western blots illustrating the effect of propentofylline (PPF), a glial inhibitor, on masseter inflammation-induced increase in GFAP (j) and IL-1β (k) in the ventral Vi/Vc transition zone. Propentofylline was injected intraperitoneally at 10 mg/kg first at 20 min before CFA, and the second PPF injection (10 mg/kg) was given 8 h after CFA. The Vi/Vc tissues were collected 1 d after CFA injection. Saline was injected as a vehicle control. Compared with naive rats, the GFAP and IL-1β immunoreactivity was increased at 1 d in rats receiving saline. The PPF treatment significantly attenuated CFA-induced increase in GFAP and IL-1β (p < 0.01; n = 3 per group.)