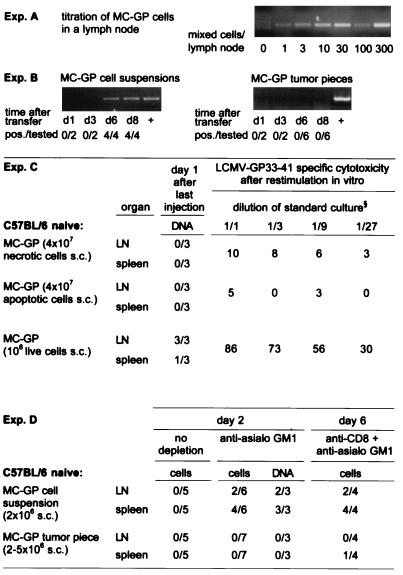
Figure 3.
Homing of MC-GP tumor cells into local lymph nodes and spleen. DNA was extracted from draining inguinal lymph nodes or spleen at the indicated time points after injection of a tumor cell suspension or after the implantation of small tumor pieces in both flanks of C57BL/6 mice. A LCMV GP-specific nested PCR was performed with the primers described in Materials and Methods. (A) The sensitivity of the assay was determined in vitro by mixing tumor cells with a constant number of 106 lymph node cells from untreated C57BL/6 mice. (B) The number of mice with positive lymph nodes over the total number of mice tested is given for each time point in each group. Lymph nodes from control C57BL/6 mice, which had not received tumor cells, and water were tested in parallel and were negative in all experiments shown. The integrity of the DNA extracted from lymph nodes was successfully tested by a perforin exon 3-specific PCR (not shown). (C) Dead tumor cells s.c. did not lead to a PCR signal or to a primed CTL response in contrast to live cells (106 s.c.). MC-GP cells (107) were either treated by freeze-thawing (necrotic, trypan blue positive, not shown) or kept on 42°C for 24 hr (apoptotic, trypan blue negative hypodiploid DNA peak in propidium iodide staining and flow cytometry, details not shown) and then injected repetitively (4 times) on alternate days in both flanks of C57BL/6 mice. One day after the last injection, DNA was prepared from spleen and from draining lymph nodes and tested by nested PCR for LCMV GP-specific DNA. Values indicate number of PCR-positive samples per total number of samples tested. At the same time point, splenocytes were restimulated in vitro for 5 days and then tested in a 51Cr-release assay. Values indicate percent specific 51Cr release as mean of three animals at the dilution of standard culture indicated. One of two comparable experiments is shown. (D) No live tumor cells could be isolated from the spleen or lymph nodes of untreated C57BL/6 mice on day 2, 4 (not shown), or 6 (not shown). Live tumor cells and GP-specific DNA could be detected in vitro after depletion of NK cells [30 μl of anti-asialo GM1 (Wako Biochemicals, Osaka) diluted in 200 μl balanced salt solution i.v. on day −1] on day 2 and after CD8 [200 μl i.p. anti-CD8 (YTS169.4.2) on days −3 and −1] plus NK depletion on day 6 after injection of MC-GP tumor cell suspensions, but not after transplantation of solid MC-GP tumor pieces. To detect live cells, lymph nodes (LN) or spleens were passed through a fine-mesh stainless steel grid, and the resulting single-cell suspension was cultured on selection medium/[0.8 mg/ml G-418 (GIBCO/BRL)]. Values indicate positive samples over total number of mice tested.