Table 2.
Ruthenium catalyzed transfer hydrogenative coupling sulfonamido-allene 1e to aldehydes 2a-2la
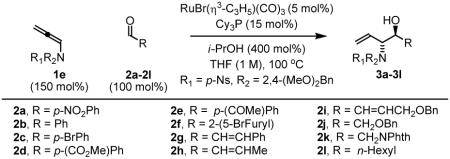 | ||
|---|---|---|
| Coupling to Aryl Aldehydes | ||
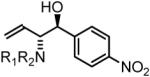 91% Yield, 3a |
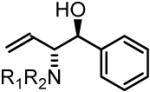 70% Yield, 3bb |
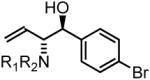 77% yield, 3c |
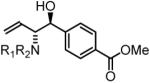 94% yield, 3d |
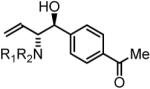 74% Yield, 3e |
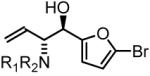 90% Yield, 3f |
| Coupling to Enals | ||
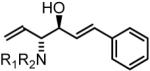 61% Yield, 3gb |
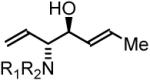 63% Yield, 3hb |
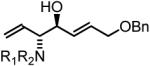 65% Yield, 3ib |
| Coupling to Aliphatic Aldehydes | ||
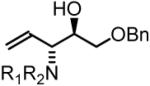 77% Yied, 3j |
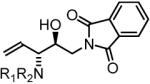 85% Yield, 3k |
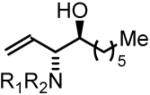 69% Yield, 3l |
In all cases, cited yields are of isolated material and represent the average of two runs. In each case, >20:1 anti-diastereoselectivity is observed, as determined by 1H NMR analysis. See Supporting Information for detailed experimental procedures.
Two equivalents of allene 1e were used.
