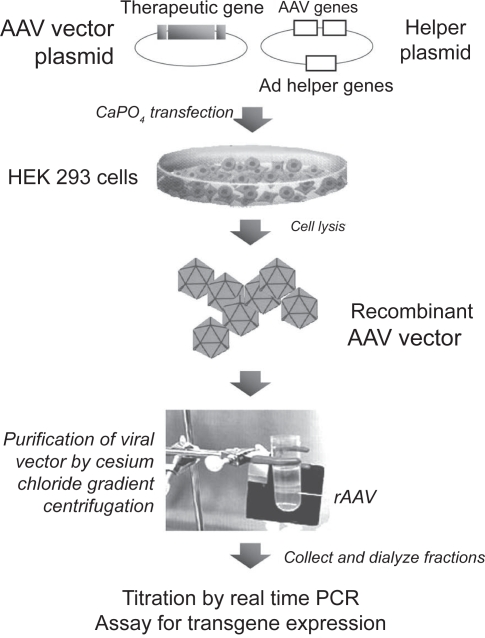
Figure 2.
Production of AAV vectors. A plasmid containing the therapeutic gene cassette (promoter+gene+polyadenylation site) cloned between the AAV terminal repeats is co-transfected into epithelial HEK293 cells together with a plasmid containing the rep and cap AAV genes and some genes from adenovirus that provide helper function. After 48 hours, cells are lysed and recombinant vector particles are purified by cesium chloride gradient centrifugation. Fractions are collected from the gradient and the number of particles containing viral genomes are quantified by real-time PCR.