Abstract
The aim of this work was to produce and characterize cetyl palmitate-based solid lipid nanoparticles (SLN) containing insulin, and to evaluate the potential of these colloidal carriers for oral administration. SLN were prepared by a modified solvent emulsification-evaporation method based on a w/o/w double emulsion. The particle size, zeta potential and association efficiency of unloaded and insulin-loaded SLN were determined and were found to be around 350 nm, negatively charged and the insulin association efficiency was over 43%. After oral administration of insulin-loaded SLN to diabetic rats, a considerable hypoglycemic effect was observed during 24 hours. These results demonstrated that SLN promote the oral absorption of insulin.
Keywords: insulin, solid lipid nanoparticles, oral administration, hypoglycemic effect, cetyl palmitate
Introduction
Insulin, a 51 amino acid peptide, has usually been administered parenterally in the treatment of diabetes mellitus (Beals and Kovach 1997). Unfortunately, injections are frequently painful, and can lead to low patient compliance. Consequently, oral delivery of insulin is expected to be an alternative route of administration to overcome compliance problems, decrease the contaminations risk and because it mimetizes in a better way the normal insulin pathway in the body after endogenous secretion (Hoffman and Ziv 1997).
Almost since the initial discovery of insulin alternative effective routes other than subcutaneous injection have been an elusive goal for many investigators (Carino and Mathiowitz 1999; Owens 2002). Oral administration of insulin has some limitations, including low oral bioavailability due to degradation in the stomach, inactivation and digestion by proteolytic enzymes in the luminal cavity, poor permeability across intestinal epithelium because of its high molecular weight and lack of lipophilicity (Carino and Mathiowitz 1999). Consequently, various approaches have been examined to overcome the delivery problems of these peptides when orally administered (Komplella and Lee 2001). Of these, carrier systems, such as liposomes (Kisel et al 2001), microemulsions (Celebi et al 2002), nanoparticles (Pan et al 2002), and microspheres (Kim et al 2002), have shown to improve the gastrointestinal absorption of peptide drugs. Also, hydrogels (Kim and Peppas 2003), azopolymer coating (Saffran et al 1991), site-specific drug delivery systems (Tozaki et al 1997), absorption enhancers (Ziv et al 1987), enzyme inhibitors (Yamamoto et al 1994) and modification of chemical structure (Asada et al 1995) have shown similar effects.
Submicron-sized particles as oral protein delivery systems protect macromolecules against the harsh environment of the gastrointestinal tract (Lowe and Temple 1994) and enhance their transmucosal transport (Tobio et al 1998). This ability of nanoparticles to enhance the transport of the encapsulated drugs has been attributed to different mechanisms such as mucoadhesion, nanoparticle internalization and permeation enhancing effect, depending on the colloidal composition. One of the advantages of nanoparticles, when administered orally, is that they can be absorbed transcellularly, not only through the membranous epithelial cells (M-cells) of the Peyer’s patches in the gut-associated lymphoid tissue (GALT) (Clark et al 2001), but also through the gut enterocytes (Hussain et al 2001). The uptake of nanoparticles carrying proteins by enterocytes has been demonstrated to be a limited but capable process (des Rieux et al 2006). Enhancing mucoadhesion properties of nanoparticles are usually explored with efficiency to promote the contact of proteins with the intestinal epithelium, increasing the concentration at the site of absorption (Garcia-Fuentes, Torres et al 2005; Tobio et al 2000).
SLN were developed at the beginning of the 1990s as an alternative carrier system to the existing traditional carriers, such as emulsions, liposomes and polymeric nanoparticles (Muller and Lucks 1996). SLN made of solid lipids (lipids being solid at room and body temperatures) are submicron colloidal carriers (50–1000 nm) dispersed either in water or in an aqueous surfactant solution (Rawat et al 2006). By definition, the lipids can be highly purified triacylglycerols, complexes acylglycerol mixtures or waxes (Souto and Müller 2007). Compared to other particulate carriers, SLN show more advantages as drug delivery system, such as a good tolerability (Muller, Maaen et al 1996), biodegradation (Muller, Ruhl et al 1996), and the possibility of production on large industrial scale (Muller et al 2000). Also, it has been report that nanoencapsulation of proteins in lipid nanoparticles has improved their bioavailability, prolonged their blood residence time and/or modified their biodistribution (Garcia-Fuentes, Torres et al 2005; Muller et al 2006).
SLN show different degradation rates by the lipolytic enzyme pancreatic lipase as a function of their composition (lipid matrix, stabilizing surfactant) and it has been demonstrated that the longer the fatty acid chains of the acylglycerols are, the slower is their degradation (Olbrich and Muller 1999). Type of surfactants can also influence degradation accelerating (eg, cholic acid sodium salt) or a hindering, degradation slowing down effect, due to steric stabilization (eg, Poloxamer 407) (Olbrich and Muller 1999). The longer the ethylene oxide chains are in the molecule, the more hindered is the anchoring of the lipase/co-lipase complex and consequently the degradation of the SLN. This knowledge can be used to adjust degradation of SLN and consequently drug release in a controlled way (Olbrich and Muller 1999). The wax cetyl palmitate seems to be suited to develop SLN intended for a controlled release of the incorporated active substances and for a frequent administration, due to a good in vitro degradation rate and a low in vivo toxicity (Lukowski et al 2000). Cetyl palmitate in SLN is arranged in a lamellar lattice structure and compounds can be stored between these layers (Lukowski et al 2000). Finally, poloxamer 407 is a stabilizing surfactant very well tolerated in vivo (Muller et al 1997).
The purpose of this work was to develop a new nanoparticulate carrier intended for the oral administration of peptides. The new carrier is composed of a lipid core aimed to protect and to control the release of insulin.
Materials and methods
Materials
Wax cetyl palmitate was provided from Gattefossé (France). Polaxomer 407 (Lutrol® micro 127) was supplied by BASF (Germany). Acetonitrile LiChrosolv HPLC grade was obtained from Merck® (Germany) and trifluoroacetic acid (TFA) from Sigma (Portugal). Dichloromethane was from Pronalab (Portugal). Human zinc-insulin (lot RS0325, 7.0 mg lyophilized human biosynthetic insulin per vial) was a generous gift from Lilly Portugal. Milli-Q-water was lab supplied.
Methods
Preparation of solid lipid nanoparticles
The method chosen for the preparation of nanoparticles was an adaptation of the w/o/w double emulsion technique (Garcia-Fuentes et al 2003; Zhang et al 2006). 200 mg of cetyl palmitate was dissolved in 4 mL of dichloromethane. 7 mg of insulin was dissolved in 0.5 mL of HCL 0.1 M. The insulin solution was added to the lipid solution and then homogenized during 30 seconds in ultra-turrax T25 (IKA-Labortechnik, Germany). The primary emulsion was poured into 25 mL of 2% poloxamer 407 solution and homogenized for additional 30 seconds. The solvent was removed and the emulsion was concentrated in rotavapor until ~10 mL.
Particle size analysis
Particle size was analyzed by photon correlation spectroscopy (PCS). Samples were diluted with Milli-Q-water to suitable concentration and the size measured with a Malvern Zetasizer 5000 (Malvern Instruments, UK). All measurements were performed in triplicate.
Zeta potential
The electrophoretic mobility was measured by Laser Doppler Anemometry (LDA) using a Malvern Zetasizer 5000 (Malvern Instruments, UK). Samples were diluted with Milli-Q-water having a conductivity adjusted to 50 μS/cm by addition of a 0.9% NaCl solution.
Transmission electron microscopy
To characterize the morphology of SLN these systems were observed by transmission electron microscopy (TEM). Samples were deposited on a grid, treated with uranil acetate and observed in a Zeiss EM 902A microscope.
Insulin association efficiency
The association efficiency (AE) was determined indirectly. The amount of insulin entrapped into SLN was calculated by the difference between the total amount used to prepare the systems and the amount of insulin that remained in the aqueous phase after SLN isolation. After preparation, aqueous SLN dispersions were centrifuged (UL 80 ultracentrifuge, rotor type 80Ti, Beckman Instruments, German) for 2 hours at 45000 rpm (corresponding to approx. 190000 × g). Insulin concentration in the supernatant was determined by HPLC (Sarmento et al 2006).
In vivo studies of insulin-loaded solid lipid nanoparticles in diabetic rats
All experiments were carried out in accordance to the Federation of European Laboratory Animal Science Association (FELASA) Guide for the Care and Use of Laboratory Animals and the European Union (Council Directive 86/609/EEC).
Male Wistar rats with 200–250 g were housed in controlled environmental conditions of temperature and relative humidity, maintained under 22 ± 2°C and 45 a 65%, respectively. The rats were fed with standard diet feed (Mucedola Top Certificate, Italy) and were provided tap water ad libitum. Lighting was on a standard 12 h on/12 h off cycle.
Diabetes was induced in rats by a single intraperitoneal injection of streptozocin (50 mg/mL in pH 4.5 citrate) at 50 mg/kg. After two weeks, rats with fasted blood glucose levels above 250 mg/dL were used for experiments. These rats were fasted for 12 h before experiments and remained fasted for 24 h during the experiment, but had free access to water ad libitum.
SLN dispersions (1.0 mL) were administered intragastrically by gavage needle to rats at insulin dose of 50 IU/kg, based on the total insulin content of the SLN. Control rats were similarly administered with equivalent volumes of insulin oral solution and empty SLN. Also, a control using subcutaneous insulin (2.5 IU/kg) was applied. Blood samples were taken from the tip of the tail vein. A 0.1 mL aliquot was collected before and 1, 2, 4, 6, 8, 10, 12, 14, 16 and 24 hours after administration.
Pharmacological availability (PA) of peroral insulin-loaded SLN was determined based on a 100% availability of the control solution administered subcutaneously to the diabetic rats at a dose of 2.5 IU of insulin/kg. Plasma glucose levels were plotted against time, and the area above the curve (AAC) below the 100% cut-off line was determined using the trapezoidal method.
Plasma glucose level was determined using the Medisense Precision Xceed Kit, (Abbot, Portugal, range 10–600 mg/dL), and expressed as a percent of the baseline plasma glucose level. Results are shown as the mean of values (± SEM) of, at least, 6 animals. One-way analysis of variance (ANOVA) was used to evaluate treatment differences. If the group by each time interaction was significantly different (P < 0.05), differences between groups were compared within a post-hoc test (S-N-K). All statistical analyses were performed with the SPSS software package (SPSS for Windows 14.0, SPSS, Chicago, USA).
Results and discussion
SLN characterization
SLN were successfully produced by a modified solvent emulsification-evaporation method based on a w/o/w double emulsion. They are considered to be stable carriers for oral administration. Poloxamer 407 was used as surfactant in the aqueous phase when preparing the insulin-loaded SLN to increase their stability. Lipid nanoparticles may suffer aggregation following incubation in gastric medium, whereas protective coating such as Poloxamer 407 or PEG diminished this phenomenon (Garcia-Fuentes et al 2003). Both unloaded and insulin-loaded SLN showed a homogenous size distribution with a mean diameter measured by PCS of 320 ± 26 nm and 361 ± 30 nm, respectively. Mean values of zeta potential were −8.0 ± 1.2 and −3.4 ± 0.2 mV for unloaded and insulin-loaded SLN, respectively. The mean diameters confirmed that the SLN produced are submicron colloidal carriers, suitable for enabling gastrointestinal absorption by M-cells on Peyer’s patches. The zeta potential values are close to the electric neutrality, but it is possible to notice a slightly less negative value for insulin-loaded SLN probably due to the deposition on the surface of the SLN of some positively charged insulin. In fact, immediately after production, the pH of insulin-colloidal SLN dispersion was found to be 3.1, lower than the isoelectric point of insulin which is 5.3 (Brange 1987), responsible for the insulin positive charge.
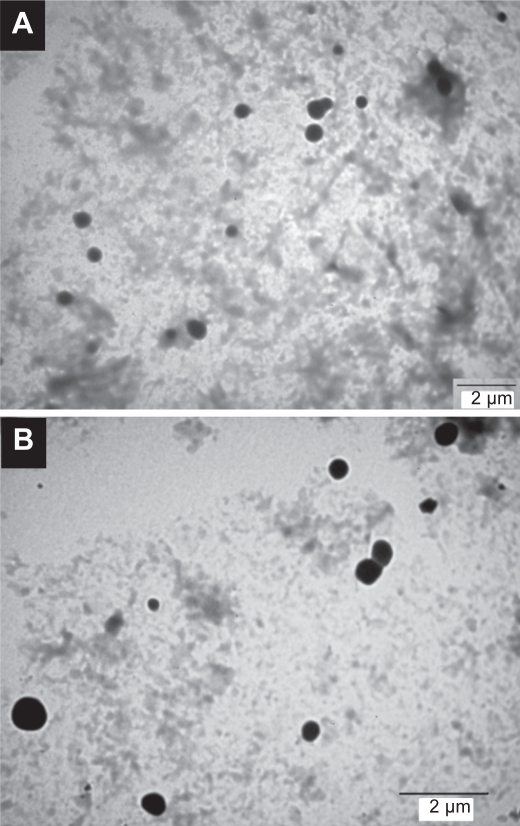
The microscopic appearance and the structural characterization of cetyl palmitate based SLN with insulin were performed using TEM (Figure 1). The particle size of unloaded and insulin-loaded SLN depicted in TEM images is in agreement with the results obtained with PCS. Furthermore, the imaging analysis showed that these particles exhibit a spherical shape, and a dense lipid matrix without aggregation. The presence of insulin on the surface of SLN was not responsible for significant agglomeration, predicting absence of aggregation after oral administration.
Figure 1.
TEM micrographs of (A) empty and (B) insulin-loaded SLN.
The AE of insulin in cetyl palmitate SLN was 43 ± 6%. This result means that approx. 56% of protein was in the dispersion medium, ie, solubilized by the surfactant molecules. It is noteworthy that being a hydrophilic molecule a much lower AE of insulin within the lipid matrix of SLN was expected. Nonetheless, the modified w/o/w double emulsion method was shown to be a suitable production procedure to achieve relatively high encapsulation for insulin.
Determination of plasma glucose levels
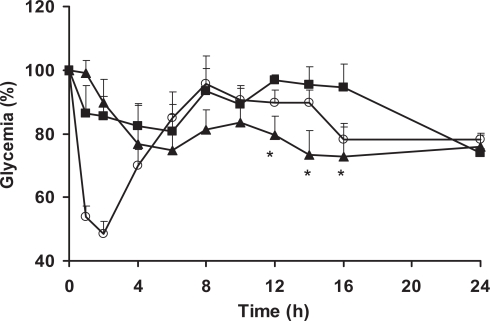
Insulin-loaded SLN were administered orally to overnight fasted diabetic rats. The reduction of the initial glucose levels versus time after intragastric insulin-loaded SLN, insulin solution and subcutaneous administration of insulin is depicted in Figure 2. The mean plasma glucose baseline value was taken as 100% level.
Figure 2.
Percentage reduction of plasma glucose concentration in diabetic rats after administration of insulin-loaded SLN 50 IU/kg (▴), subcutaneous injection of insulin 2.5 IU/Kg (○) and oral insulin solution 50 IU/kg (▪). Data represents the mean ± SEM, n = 6 per group. *Statistically significant differences from oral insulin solution (p < 0.05).
After subcutaneous administration of insulin solution (2.5 IU/Kg), glycemia decreased significantly by 45% after 1 h to a maximal decrease of 55% after 2 h. This effect was maintained up to 4 h and control values were reached again after 6 h. Administration of intragastric aqueous insulin solution, at a dose of 50 IU/kg, was responsible for a minimum decrease, less that 20%, on the glycemia obtained after 6 h. Although the absence of physiologic effect after administration of insulin solution was expected, the absorption of a small fraction of insulin prior to its degradation could not be discarded. Insulin introduced in the lumen of the rat duodenum and colon was observed to be rapidly internalized by the epithelial cells and transferred through a transcytotic pathway via the Golgi apparatus to the interstitial space from which it reached the blood circulation (Ziv and Bendayan 2000). This uptake of insulin was attributed through binding to specific insulin receptors in intestinal enterocytes. Then the exogenous insulin induces significant decreases in plasma glucose levels which lasts for several hours (Bendayan et al 1994; Ziv and Bendayan 2000).
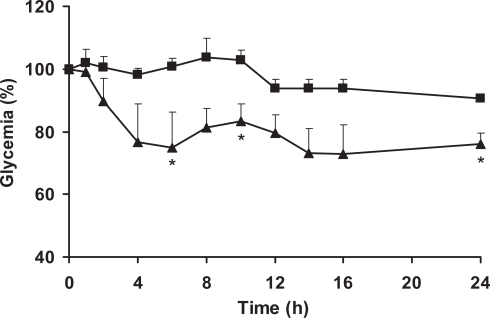
Insulin-loaded SLN decreased glycemia by comparison with rats treated with oral insulin solution (Figure 2) and empty nanoparticles (Figure 3). This hypoglycemic effect was observed to occur in a biphasic way, with an initial peak between 4 and 8 h with a decrease of 25% of the initial glucose level and later after 12 h of assay prolonged for up to 24 h. This may be related with the typical biphasic drug release pattern with an initial burst and prolonged release from SLN, characterized by a microcrystal matrix structure (Hu et al 2002; Wissing et al 2004; Souto 2005). Such biphasic pattern can originate an immediate release of insulin but also retain a significant fraction of the drug entrapped into the lipid matrix during prolonged time. Insulin released from SLN in the intestinal lumen is able to be directly internalized as discussed above, being the first responsible for the physiological effect. Then, after arrival to the appropriate sites for nanoparticle uptake in the posterior ileum (Jung et al 2000; Cui et al 2006), SLN are able to be absorbed (Garcia-Fuentes, Prego et al 2005), resulting in significant hypoglycemic effect compared with oral insulin solution administration. Afterwards, it can be postulated that SLN undergo physiological degradation and the insulin enter into the blood circulation. It is generally accepted that nanoparticles with hydrophobic surfaces such as SLN, are taken up more extensively by the intestinal epithelium than those with hydrophilic surfaces (Eldridge et al 1990). Thus, the uptake of nanoparticles with lipid matrix is potentially facilitated. Also, the bioadhesive properties of lipids can lead to a gradient diffusion of insulin from the high concentrations in the SLN matrix towards the intestinal cells. The association of both mechanisms has been related with the prolonged physiologic affect of proteins after oral administration (Damge et al 1997; Lin et al 2007).
Figure 3.
Percentage reduction of plasma glucose concentration in diabetic rats after administration of an insulin-loaded SLN 50 IU/kg (▴) and empty SLN (▪).Data represents the mean ± SEM, n = 6 per group. *Statistically significant differences from negative empty nanoparticle (p < 0.05).
As shown in Table 1, the relative pharmacological bioavailability of insulin was 5.0% when administered into SLN and 1.6% in oral insulin solution. These overall results suggested that SLN could protect insulin from degradation and enhance intestinal absorption of insulin.
Table 1.
Parameters for plasma glucose levels and relative pharmacological bioavailability Data represents the mean ± SD, n = 6 per group
| Insulin subcutaneous | Insulin-loaded SLN | Oral insulin solution | |
|---|---|---|---|
| Insulin dose (IU/kg) | 2.5 | 50.0 | 50.0 |
| Cmin (%) | 48.6 ± 3.9 | 73.2 ± 7.7 | 80.8 ± 8.3 |
| Tmin (h) | 2 | 14 | 6 |
| AAC | 478 ± 125 | 484 ± 196 | 260 ± 37 |
| PA%a | - | 5.1 ± 3.1* | 1.6 ± 0.7 |
Cmin, minimum plasma glucose concentration (% of initial); Tmin, time to Cmin; AAC, area above the plasma glucose levels time curves; PA%, relative pharmacological bioavailability.
Based on AAC for subcutaneous administration (SC).
Statistically significant differences from oral insulin solution control (p < 0.05).
Conclusion
The attempts to develop an insulin-loaded SLN formulation for oral administration produced nanoparticles with spherical shape, slight negative zeta potential values and good association efficiency. The plasma glucose levels of rats after oral administration of insulin-loaded SLN were lower that those obtain after administration of oral insulin solution and empty SLN up to 24 h. The solid matrix of SLN was able to partially protect insulin against chemical degradation in the gastrointestinal tract and to promote the intestinal absorption of insulin. In conclusion, SLN were found to be suitable carrier systems for the administration of insulin through the oral route. This study may contribute for the development of an optimized oral insulin formulation.
Acknowledgments
The authors would like to thank to Joel Fonseca and Ana Margarida Silva for their collaboration during in vivo studies. The insulin supply by Lilly Portugal is highly appreciated.
References
- Asada H, Douen T, Waki M, et al. Absorption characteristics of chemically modified-insulin derivates without fatty acids in the small and large intestine. J Pharm Sci. 1995;84:682–7. doi: 10.1002/jps.2600840604. [DOI] [PubMed] [Google Scholar]
- Beals JM, Kovach P. Crommelin DJA, Sindelar RD. Pharmaceutical biotechnology. Netherlands: Harwood Academic Publishers; 1997. Insulin; pp. 229–39. [Google Scholar]
- Bendayan M, Ziv E, Gingras D, et al. Biochemical and morphocytochemical evidence for the intestinal absorption of insulin in control and diabetic rats. Comparison between the effectiveness of duodenal and colon mucosa. Diabetologia. 1994;37:119–26. doi: 10.1007/s001250050081. [DOI] [PubMed] [Google Scholar]
- Brange J. Galenics of insulin: the physico-chemical and pharmaceutical aspects of insulin and insulin preparations. Springer-Verlag; 1987. [Google Scholar]
- Carino GP, Mathiowitz E. Oral insulin delivery. Adv Drug Deliv Rev. 1999;35:249–57. doi: 10.1016/s0169-409x(98)00075-1. [DOI] [PubMed] [Google Scholar]
- Celebi N, Turkyilmaz A, Gonul B, et al. Effects of epidermal growth factor microemulsion formulation on the healing of stress-induced gastric ulcer in rats. J Control Release. 2002;83:197–210. doi: 10.1016/s0168-3659(02)00198-0. [DOI] [PubMed] [Google Scholar]
- Clark MA, Jepson MA, Hirst BH. Exploiting M cells for drug and vaccine delivery. Adv Drug Deliv Rev. 2001;50:81–106. doi: 10.1016/s0169-409x(01)00149-1. [DOI] [PubMed] [Google Scholar]
- Cui F, Shi K, Zhang L, et al. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: Preparation, in vitro characterization and in vivo evaluation. J Control Release. 2006;114:242–50. doi: 10.1016/j.jconrel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Damge C, Vrancks H, Balschmidt P, et al. Poly(alkyl cyanoacrylate) nanospheres for oral administration of insulin. J Pharm Sci. 1997;86:1407–500. doi: 10.1021/js970124i. [DOI] [PubMed] [Google Scholar]
- des Rieux A, Fievez V, Garinot M, et al. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Eldridge JH, Hammond CJ, Meulbroek JA, et al. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the peyer’s patches. J Control Release. 1990;11:205–14. [Google Scholar]
- Garcia-Fuentes M, Prego C, Torres D, et al. A comparative study of the potential of solid triglyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur J Pharm Sci. 2005;25:123–33. doi: 10.1016/j.ejps.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuentes M, Torres D, Alonso MJ. Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Colloid Surface B. 2003;27:159–68. [Google Scholar]
- Garcia-Fuentes M, Torres D, Alonso MJ. New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int J Pharm. 2005;296:122–32. doi: 10.1016/j.ijpharm.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Hoffman A, Ziv E. Pharmacokinetic considerations of new insulin formulations and routes of administration. Clin Pharmacokinet. 1997;33:285–301. doi: 10.2165/00003088-199733040-00004. [DOI] [PubMed] [Google Scholar]
- Hu FQ, Yuan H, Zhang HH, et al. Preparation of solid lipid nanoparticles with clobetasol propionate by a novel solvent diffusion method in aqueous system and physicochemical characterization. Int J Pharm. 2002;239:121–8. doi: 10.1016/s0378-5173(02)00081-9. [DOI] [PubMed] [Google Scholar]
- Hussain N, Jaitley V, Florence AT. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50:107–42. doi: 10.1016/s0169-409x(01)00152-1. [DOI] [PubMed] [Google Scholar]
- Jung T, Kamm W, Breitenbach A, et al. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm. 2000;50:147–60. doi: 10.1016/s0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- Kim B, Peppas NA. In vitro release behavior and stability of insulin in complexation hydrogels as oral drug delivery carriers. Int J Pharm. 2003;266:29–37. doi: 10.1016/s0378-5173(03)00378-8. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Choi HK, Suh SP, et al. Pharmacokinetic and pharmacodynamic evaluation of cyclosporine an o/w-emulsion and microsphere formulations in rabbits. Eur J Pharm Sci. 2002;15:497–502. doi: 10.1016/s0928-0987(02)00048-9. [DOI] [PubMed] [Google Scholar]
- Kisel MA, Kulik LN, Tsybovsky IS, et al. Liposomes with phosphatidylethanol as a carrier for oral delivery of insulin: studies in the rat. Int J Pharm. 2001;216:105–14. doi: 10.1016/s0378-5173(01)00579-8. [DOI] [PubMed] [Google Scholar]
- Komplella UB, Lee VHL. Delivery systems for penetration enhancement of peptide and protein drugs: design considerations. Adv Drug Deliv Rev. 2001;46:211–45. doi: 10.1016/s0169-409x(00)00137-x. [DOI] [PubMed] [Google Scholar]
- Lin YH, Mi FL, Chen CT, et al. Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules. 2007;8:146–52. doi: 10.1021/bm0607776. [DOI] [PubMed] [Google Scholar]
- Lowe PL, Temple CS. Calcitonin and insulin in isobutilcyanoacrylate nanocapsules: Protection against proteases and effect on intestinal absorption. J Pharm Pharmacol. 1994;46:547–52. doi: 10.1111/j.2042-7158.1994.tb03854.x. [DOI] [PubMed] [Google Scholar]
- Lukowski G, Kasbohm J, Pflegel P, et al. Crystallographic investigation of cetylpalmitate solid lipid nanoparticles. International Journal of Pharmaceutics. 2000;196:201–5. doi: 10.1016/s0378-5173(99)00421-4. [DOI] [PubMed] [Google Scholar]
- Muller RH, Lippacher A, Gohala S. Solid lipid nanoparticles (SLN) as carrier system for the controlled release of drugs. In: Wise DL, editor. Handbook of Pharmaceutical controlled release technology. Marcel Dekker; 2000. pp. 377–91. [Google Scholar]
- Muller RH, Lucks JS. Azneistoffträger aus festen Lipidteilchen feste Lipid Nanosphären (SLN) European Patent 0605497; Germany: 1996. [Google Scholar]
- Muller RH, Maaen S, Weyhers H, et al. Cytotoxicity of magnetite-loaded polylactide, polylactide/glycolide particles and solid lipid nanoparticles. Int J Pharm. 1996;138:85–94. [Google Scholar]
- Muller RH, Ruhl D, Runge S. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. Int J Pharm. 1996;144:115–21. [Google Scholar]
- Muller RH, Ruhl D, Runge S, et al. Cytotoxicity of Solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharm Res. 1997;14:458–62. doi: 10.1023/a:1012043315093. [DOI] [PubMed] [Google Scholar]
- Muller RH, Runge S, Ravelli V, et al. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm. 2006;317:82–9. doi: 10.1016/j.ijpharm.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Olbrich C, Muller RH. Enzymatic degradation of SLN—effect of surfactant and surfactant mixtures. Int J Pharm. 1999;180:31–9. doi: 10.1016/s0378-5173(98)00404-9. [DOI] [PubMed] [Google Scholar]
- Owens DR. New horizons—Alternative routes for insulin therapy. Nat Rev Drug Discov. 2002;1:529–40. doi: 10.1038/nrd836. [DOI] [PubMed] [Google Scholar]
- Pan Y, Li YJ, Zhao HY, et al. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 2002;249:139–47. doi: 10.1016/s0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- Rawat M, Singh D, Saraf S, et al. Nanocarriers: Promising Vehicle for Bioactive Drugs. Biol Pharm Bull. 2006;29:1790–8. doi: 10.1248/bpb.29.1790. [DOI] [PubMed] [Google Scholar]
- Saffran M, Field JB, Pena J, et al. Oral insulin in diabetic dogs. J Endocrinol. 1991;131:267–78. doi: 10.1677/joe.0.1310267. [DOI] [PubMed] [Google Scholar]
- Sarmento B, Ribeiro A, Veiga F, et al. Development and validation of a rapid reversed-phase HPLC method for the determination of insulin from nanoparticulate systems. Biomed Chromatogr. 2006;20:898–903. doi: 10.1002/bmc.616. [DOI] [PubMed] [Google Scholar]
- Souto EB.2005SLN and NLC for topical delivery of antifungalsPh.D ThesisFreie Universitat Berlin; Berlin [Google Scholar]
- Souto EB, Müller RH. Lipid nanoparticles (SLN and NLC) for drug delivery. In: Domb AJ, Tabata Y, Kumar MNVR, et al., editors. Nanoparticles for pharmaceutical applications. American Scientific Publishers; 2007. pp. 103–22. [Google Scholar]
- Tobio M, Gref R, Sanchez A, et al. Stealth PLA-PEG Nanoparticles as Protein Carriers for Nasal Administration. Pharm Res. 1998;15:270–5. doi: 10.1023/a:1011922819926. [DOI] [PubMed] [Google Scholar]
- Tobio M, Sanchez A, Vila A, et al. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloid Surface B. 2000;18:315–23. doi: 10.1016/s0927-7765(99)00157-5. [DOI] [PubMed] [Google Scholar]
- Tozaki H, Komoike J, Tada C, et al. Chitosan capsules for colo-specific drug delivery: improvement of insulin absorption from the rat colon. J Pharm Sci. 1997;86:1016–21. doi: 10.1021/js970018g. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Taniguchi T, Rikyuu K, et al. Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm Res. 1994;11:1496–500. doi: 10.1023/a:1018968611962. [DOI] [PubMed] [Google Scholar]
- Zhang N, Ping Q, Huang G, et al. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int J Pharm. 2006;327:153–9. doi: 10.1016/j.ijpharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Ziv E, Bendayan M. Intestinal absorption of peptides through the enterocytes. Microsc Res Tech. 2000;49:346–52. doi: 10.1002/(SICI)1097-0029(20000515)49:4<346::AID-JEMT3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ziv E, Lior O, Kidron M. Absorption of protein via the intestinal wall. A quantitative model. Biochem Pharmacol. 1987;36:1035–9. doi: 10.1016/0006-2952(87)90411-4. [DOI] [PubMed] [Google Scholar]