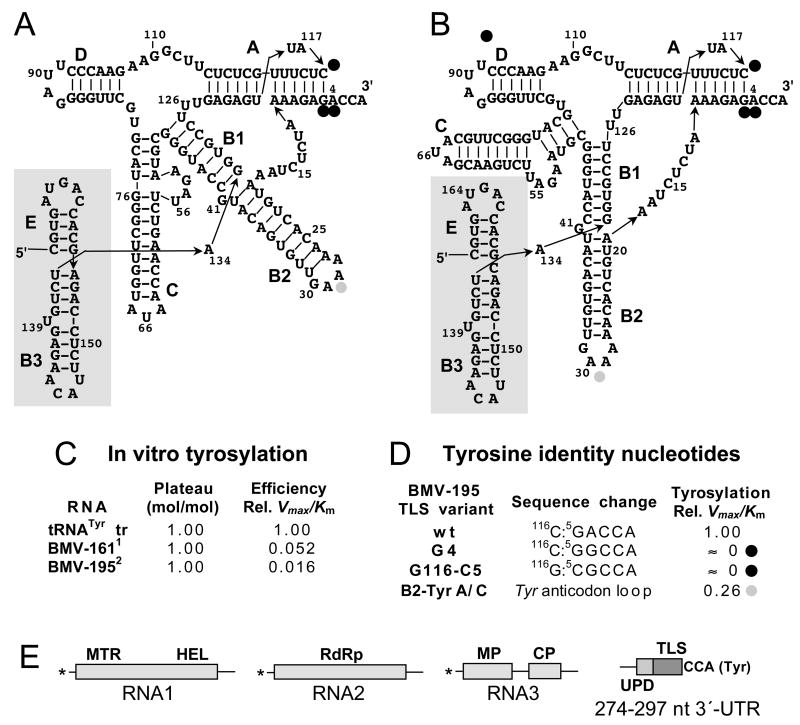
Fig. 3. The tyrosine-specific BMV TLS.
A, B Sequence of the 133-nt-long TLS of BMV RNA3 and adjacent upstream stem-loops. The structure in (A) was originally proposed by (Rietveld et al., 1983), who suggested that arm C with its AUA tyrosyl anticodon serves as the analog of the anticodon domain of tRNA. The current structural model proposed by (Fechter et al., 2001a) in (B) shows arm B2 as the analog of the anticodon. The major tyrosine identity elements are present at the 3′-end of the acceptor stem helix, and only minor tyrosine identity is contributed by the B2 loop (Fechter et al., 2001a). Stems C and D can be considered as insertions into the basic tRNA-like structure; the terminal AUA loop of stem C serves as the core element of the minus strand promoter, and stem D is absent from some bromoviral TLSs. Stems B3 and E (shaded) are outside the core TLS, but mutations in B3 impair tyrosylation (see text). C. Comparison of in vitro tyrosylation by yeast TyrRS of two forms of BMV RNA with an in vitro-generated yeast tRNATyr; 1 BMV-161 is a 161 nt-long 3′-fragment (Ω161) produced from RNA4 by limited ribonuclease cleavage (Perret et al., 1989); 2 BMV-195 is a 195 nt RNA3 transcript (Fechter et al., 2001a). D. Contributions to tyrosine identity by nucleotides at the end of the acceptor stem helix, and by the B2 loop, studied with yeast TyrRS (Fechter et al., 2001a). E. Diagram of the tripartite BMV genome with domains labeled as in Fig. 1. The major domains in the 3′-UTR are the TLS and the upstream domain (UPD), both of which are strongly conserved among the three genomic RNAs. The core minus strand promoter resides in stem C within the TLS; the 3′-UTR serves as a translational enhancer, but the responsible domain has not been identified (see text).