Scheme 1.
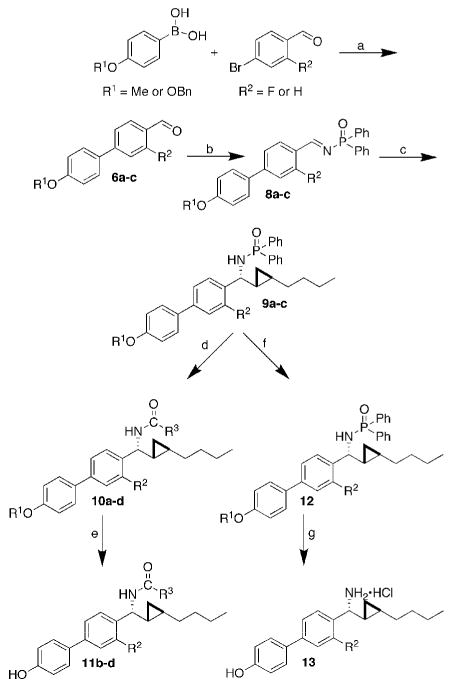
Overview of the synthesis of six new biphenyl substituted C-cyclopropylalkylamides. Reagents and conditions: a, DAPCy (2 mol%), Cs2CO3, dioxane, r.t; b, Ph2P(O)NH2, TiCl4, Et3N, r.t; c, i) Cp2Zr(H)Cl, 1-hexyne, CH2Cl2, ii) Me2Zn, toluene, -78 to 0°C, iii) μW 100 °C, 300 W, 10 min, iv) CH2I2, r.t.; d, (1) HCl, MeOH, (2) R3COCl, DMAP, iPr2EtN, r.t.; e, H2, Pd(OH)2/C, THF, MeOH, r.t.; f, H2, Pd(OH)2/C, THF, MeOH, r.t.; g, HCl, MeOH. Percent yields: 6a, 70; 6b, 88; 6c, 50; 8a, 47; 8b, 67; 8c, 40; 9a, 30; 9b, 68; 9c, 40; 10a, 61; 10b, 57; 10c, 22; 10d, 75; 11b, 69; 11c, 92; 11d, 94; 12, 80; 13, 43. R1 = Me, R2 = F (6a, 8a, 9a); R1 = Bn, R2 = F (6b, 8b, 9b); R1 = Bn, R2 = H (6c, 8c, 9c); R1 = Me, R2 = F, R3 = Ph (10a); R1 = H, R2 = F, R3 = Ph (10b, 11b); R1 = H, R2 = H, R3 = Ph (10c, 11c); R1 = H, R2 = F, R3 = Me (10d, 11d); R2 = F (12, 13).
