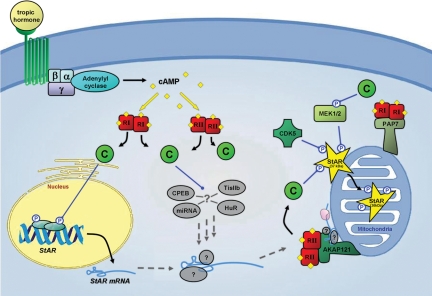
Figure 2.
A model illustrating post-transcriptional and post-translational regulation of StAR. Tropic hormone activation initiates multiple signal transduction pathways known to induce StAR gene expression. Central to these pathways is the second messenger cAMP that binds to the regulatory subunits (RI and RII) of PKA resulting in the release of the active catalytic subunits (C). In addition to directing StAR gene transcription, it is predicted that PKA and other signaling events are likely to coordinate the post-transcriptional regulation of StAR mRNA, potentially through miRNAs and mRNA-binding proteins such as Tis11b, HuR and CPEB. The expression of StAR mRNA as a result of this process appears to be enhanced by A-kinase anchor protein (AKAP)121, which may recruit StAR mRNA, possibly in complex with other proteins, to the outer mitochondrial membrane, allowing it to be translated and activated on site. AKAP121 also tethers type II PKA to the mitochondria, which appears to serve a role in enhancing the activation of StAR at the outer mitochondrial membrane through the phosphorylation of Ser195 in StAR. Similarly, acyl-co-enzyme A-binding domain containing 3 (PAP7; also, ABCD3) has been demonstrated to bind type I PKA at the outer mitochondrial, also increasing steroidogenesis. The pool of AKAP-tethered PKA is likely to serve in activating mitochondrial kinase MEK1/2, which has recently been shown to activate StAR by phosphorylating Ser232. Furthermore, StAR activity in Leydig cells has been linked to its association with and phosphorylation by Cdk5; however, the specific target on StAR for this kinase remains unknown. These post-translational modifications to StAR may serve to enhance its stability or its ability to interact with other proteins necessary for cholesterol transport. Alternatively, these events may serve to prolong the duration it takes for StAR to be withdrawn into the mitochondria. The N-terminal region of full-length (37 kDa) StAR targets the protein for importation into the mitochondria and is proteolytically cleaved following StAR’s translocation. Thus, although the C-terminal domain (30 kDa) of StAR possesses the intrinsic capacity to promote cholesterol transfer, it is possible that the steroidogenic potential of StAR is maximized by events that cause it to dwell longer at the outer mitochondrial membrane in contact with other proteins known to promote steroidogenesis. (Figures and dashed arrows depicted in gray represent putative targets and interactions.)