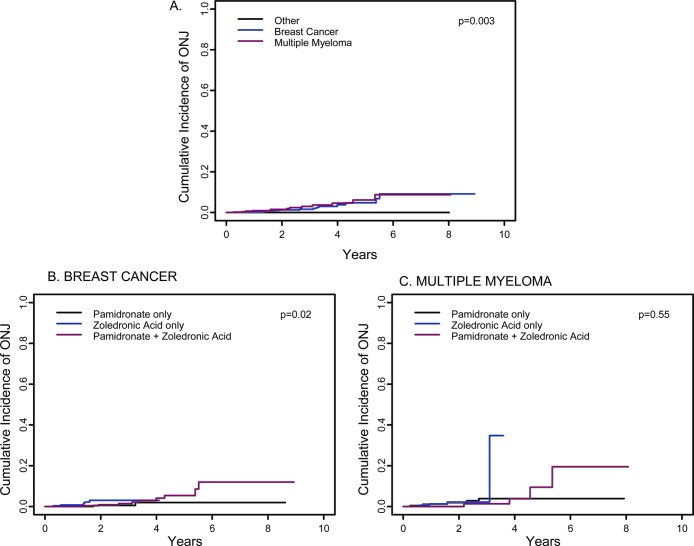
FIG. 3.
Estimate of cumulative incidence of ONJ. (A) Cumulative incidence of ONJ in 3994 patients with breast cancer (blue line), multiple myeloma (red line), or other disorders (black line). The estimated cumulative incidence of ONJ at 3 yr was 1.6% (95% CI: 0.005–0.027) in breast cancer, 3% (95% CI: 0.009–0.050) in multiple myeloma, and 0% in other disorders (p = 0.003). The comparable data at 5 yr are 4.8% (95% CI: 1.7–7.9%); 6.2% (95% CI: 1.9–10.4%); and 0%. (B and C) Cumulative incidence of ONJ at 3 yr in breast cancer and multiple myeloma patients analyzed by type of bisphosphonate used. In the breast cancer group at 3 yr (B), the estimated cumulative incidence of ONJ is 0.5% (95% CI: 0.000–0.016) with pamidronate, 3.0% (95% CI: 0.000–0.058) with zoledronic acid, and 1.4% (95% CI: 0.000–0.031) with pamidronate followed by zoledronic acid (p = 0.02). The comparable data for the breast cancer group at 5 yr are 2% (95% CI: 0–4.9%), inadequate numbers for zoledronic acid, and 5.4% (95% CI: 1.1–9.7%). The differences are significant at 5 yr. In the multiple myeloma group, the estimated cumulative incidence of ONJ or maxilla at 3 yr is 3.9% (95% CI: 0.004–0.074) with pamidronate, 2.2% (95% CI: 0.000–0.047) with zoledronic acid, and 1.3% (95% CI: 0.000–0.039) with pamidronate followed by zoledronic acid (p = 0.55). The comparable data for the multiple myeloma group at 5 yr are 3.9% (95% CI: 0.4–7.4%), inadequate for zoledronic acid, and 9.4% (95% CI: 0–21.7%).