Abstract
Early invasions of the North American shore occurred mainly via deposition of ballast rock, which effectively transported pieces of the intertidal zone across the Atlantic. From 1773–1861, >880 European ships entered Pictou Harbor, Nova Scotia, as a result of emigration and trade from Europe. The rockweed Fucus serratus (1868) and the snail Littorina littorea (≈1840) were found in Pictou during this same period. With shipping records (a proxy for propagule pressure) to guide sampling, we used F. serratus as a model to examine the introductions because of its relatively low genetic diversity and dispersal capability. Microsatellite markers and assignment tests revealed 2 introductions of the rockweed into Nova Scotia: 1 from Galway (Ireland) to Pictou and the other from Greenock (Scotland) to western Cape Breton Island. To examine whether a high-diversity, high-dispersing species might have similar pathways of introduction, we analyzed L. littorea, using cytochrome b haplotypes. Eight of the 9 Pictou haplotypes were found in snails collected from Ireland and Scotland. Our results contribute to a broader understanding of marine communities, because these 2 conspicuous species are likely to be the tip of an “invasion iceberg” to the NW Atlantic from Great Britain and Ireland in the 19th Century.
Keywords: Fucus serratus, Littorina littorea, propagule pressure
Factors responsible for the establishment and expansion of introduced species are being investigated in many different ecosystems, including in marine communities where biological invasions are occurring at an apparently unprecedented rate (1, 2). Propagule pressure, the number of individuals released into a new habitat, is emerging as a significant factor in successful invasions (e.g., refs. 3–5), which highlights the importance of studying invasion vectors (6). Here, we examine the role of vectors and propagule pressure in the ≈19th Century establishment of 2 European species in North America: the rockweed Fucus serratus and the herbivorous snail Littorina littorea. Both are conspicuous, cooccurring members of intertidal and shallow subtidal communities in northern Europe and, more recently, North America (7–9).
We focus first on F. serratus, because its limited natural dispersal, comparatively low genetic diversity, and phylogeographic history in its native European range (10, 11) permit a focused investigation of potential source locations. We then shift to L. littorea, which differs substantially from F. serratus because of high dispersal and high genetic diversity throughout its native European range (9).
Both species were first recorded in North America near Pictou, Nova Scotia: L. littorea in the 1840s (12) and F. serratus in the 1860s (13). By 1880, L. littorea had advanced southward to Long Island Sound, NY. Its southern limit today is at Lewes, DE, with a northern limit at Red Bay, Labrador (14, 15). L. littorea was last present in Iceland at 1.1 My BP and is unknown from Greenland (16); therefore, a stepping-stone invasion across the North Atlantic appears unlikely for this species, as opposed to the indigenous littorines, L. saxatilis and L. obtusata, which are believed to have recolonized North America after deglaciation via this natural, stepping-stone expansion pathway (15, 17). F. serratus remains restricted to the Canadian Maritimes (ref. 7; Fig. 1). It is not found in Greenland, but genetic and historical analyses revealed that it was introduced to Iceland from Norway in the early 19th Century (18).
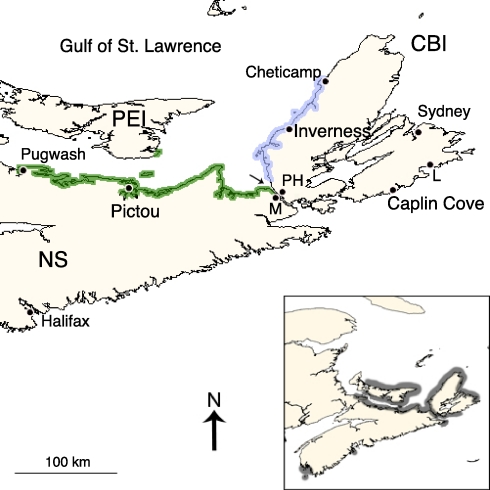
Fig. 1.
Distribution of the European seaweed Fucus serratus in 1903 (19; blue and green border) and today (Inset, gray border) in the Canadian Maritimes. In 1903, F. serratus occurred from Pugwash to Mulgrave (M) on the Strait of Canso (arrow) between Cape Breton Island (CBI) and mainland Nova Scotia (NS), the tip of Prince Edward Island (PEI), and southward from Cheticamp to an area near Port Hawkesbury (PH). Our molecular results indicate that this distribution represents a convergence at the Strait of Canso of 2 different introductions. The shore near the French Fortress (1713–1758) of Louisbourg (L) remains uncolonized. Secondary (20th Century) colonization sites in mainland NS are shown in Inset.
Although the ultimate source for both introductions is Europe, narrowing this region within Europe depends on being able to match genetic signatures between locations, which, in turn, depends on both the level of genetic variation and the geographic sampling density, a task that is easier for F. serratus than L. littorea. Coyer et al. (10) proposed the source of a single Nova Scotian population of F. serratus to be the Brittany area of western France (but sampling in Great Britain and Ireland was minimal); whereas the source of L. littorea has been attributed to vectors originating from Scandinavia (via Vikings) or 17th–19th Century Europeans (reviewed in refs. 9 and 15). To determine the original source(s) for both species, we analyzed historical shipping records between European ports and Nova Scotia to define the magnitude and sources of vectors to Pictou. This information guided further sampling and biological analyses.
Our aims were to: (i) resolve the long-standing questions of the European source(s) of F. serratus and L. littorea and (ii) test the degree to which high-frequency pathways of transport (i.e., propagule pressure proxies) correlated with the successful establishment of these nonindigenous species. In doing so, this study refocuses attention on how strongly biological invasions linked to 19th century world events have affected intertidal community structure in the northwestern Atlantic.
Results
Assignment to Source Populations.
Fucus serratus.
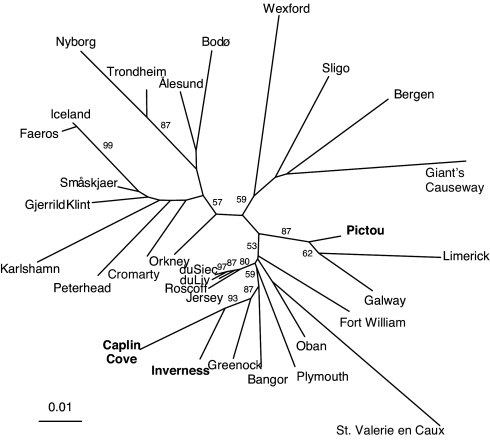
The neighbor-joining tree (Fig. 2) places 3 Nova Scotian populations of F. serratus into 2 different clades. Inverness (N.S.) and Caplin Cove (N.S.) (Figs. 1 and 2) clustered together with Greenock (Scotland) (bootstrap = 87%); whereas, Pictou clustered with Galway and Limerick (Ireland) (bootstrap = 87%). The distinct clustering of the Nova Scotian populations strongly suggests that there were at least 2 independent introductions to Nova Scotia from Scotland and Ireland. A Bayesian assignment test (20) showed that Greenock, Scotland, was the most likely source for the Inverness and Caplin Cove populations [Fig. 2, Table 1, supporting information (SI) Table S1, and Table S2]. In contrast, the Pictou population was assigned to Galway, Ireland (Fig. 2, Table 1, Table S1, and Table S2) and had similar allelic characteristics (Table S2).
Fig. 2.
Neighbor-joining tree based on microsatellite analysis supports 2 distinct introductions of F. serratus into Nova Scotia, 1 from western Ireland and 1 from the Clyde area (Greenock) of Scotland. Nova Scotian sites are in bold type.
Table 1.
Log-likelihood assignment scores for F. serratus following Rannala and Mountain (20)
| Pictou (Nova Scotia) |
Inverness (Nova Scotia) |
Caplin Cove (Nova Scotia) |
||||||
|---|---|---|---|---|---|---|---|---|
| Coming from | Likelihood score | Assignment probability | Coming from | Likelihood score | Assignment probability | Coming from | Likelihood score | Assignment probability |
| Galway (Ireland) | 123.578 | 100.00 | Greenock (Scotland) | 137.683 | 100.00 | Greenock | 126.469 | 100.00 |
| Jersey (CI) | 155.36 | 0.0 | Bangor (NI) | 158.188 | 0.0 | Oban (S) | 145.879 | 0.0 |
Table S1 includes assignment scores for all sites. CI, Channel Islands; I, Ireland; NI, Northern Ireland; S, Scotland.
Littorina littorea.
This study adds 3 North American and 31 European cytochrome b haplotypes to those described (9) for a grand total of 25 North American and 117 European haplotypes. Here, we focus on shared haplotypes between different areas of Europe and Pictou, N.S. (n = 8 haplotypes) and Nova Scotia as a whole (n = 11 haplotypes) because of early reports (12, 15) of L. littorea in these places. The other 14 North American haplotypes occur at low frequency (Dataset S1); statistical analysis predicts they are shared with yet undiscovered European haplotypes (9).
Eight of the 9 Pictou haplotypes are shared with Europe and are also the highest frequency North American haplotypes (Dataset S1); the ninth Pictou haplotype is basal to a Scottish haplotype (Dataset S1). Only 3 of the 8 shared haplotypes in Pictou were found in Scandinavia, whereas 6 of 8 were shared with midcontinental Europe (The Netherlands, Belgium, Atlantic France, and Spain), and all 8 were shared with Great Britain and Ireland, as well as just Scotland and Ireland. When all shared European haplotypes in Nova Scotia (n = 11) were included for comparison, they matched most closely with Great Britain and Ireland rather than Scandinavia or midcontinental Europe (Table 2; Fig. S1). However, because sampling effort was unequal across regions, we performed Monte Carlo (MC) standardizations using both LISV and Chao2 analysis (21) to explore the estimated number of total haplotypes shared between Pictou or Nova Scotia and each European region (Table 2). Both MC analyses found the same pattern as the original analysis i.e., estimates for shared haplotypes between Pictou or Nova Scotia and European regions were highest in Great Britain/Ireland (Scotland and Ireland) and lowest in Scandinavia (Table 2). The Chao2 estimator predicted a few additional shared haplotypes between all European regions and Pictou or Nova Scotia except in Scandinavia, further suggesting that North American L. littorea populations did not originate from Scandinavia.
Table 2.
Littorina littorea haplotypes in Pictou, N.S., and Nova Scotia as a whole vs. different regions of Europe [Great Britain and Ireland (Ireland, Scotland, Wales, and England), Scandinavia (Denmark, Sweden, and Norway), midcontinental Europe (Belgium, Netherlands, France, and Spain), and All Europe (except Britain/Ireland)]
| European (EUR) region* | Haplotypes shared with EUR region (no.) | Haplotypes shared with EUR region (proportion) | Shared individuals within EUR region (no.) | Haplotypes shared with EUR region (adjusted no.; LISV analysis) | Haplotypes shared with EUR region (adjusted proportion; LISV analysis) | Estimated haplotypes shared within EUR region (no.; mean Chao2) |
|---|---|---|---|---|---|---|
| Pictou, N.S. (n = 36; total shared haplotypes = 8) | ||||||
| Britain and Ireland | 8 | 1.00 | 72 | 7 | 0.88 | 9 |
| Scotland and Ireland | 8 | 1.00 | 66 | 7 | 0.88 | 9 |
| Scandinavia | 3 | 0.38 | 37 | 3 | 0.38 | 3 |
| Midcontinental EUR | 6 | 0.75 | 35 | 6 | 0.75 | 8 |
| EUR except Britain and Ireland | 6 | 0.75 | 72 | 4 | 0.50 | 8 |
| Nova Scotia (n = 61; total shared haplotypes = 11) | ||||||
| Britain and Ireland | 11 | 1.00 | 75 | 8 | 0.73 | 14 |
| Scotland and Ireland | 10 | 0.91 | 68 | 8 | 0.73 | 14 |
| Scandinavia | 3 | 0.27 | 37 | 3 | 0.27 | 3 |
| Midcontinental EUR | 7 | 0.64 | 36 | 7 | 0.64 | 12 |
| EUR except Britain and Ireland | 7 | 0.64 | 73 | 5 | 0.45 | 12 |
Both Pictou and Nova Scotia shared many haplotypes with Great Britain/Ireland and few with Scandinavia. A similar pattern was found following Monte Carlo sample standardization through LISV analysis and Chao2 estimation (SI Text).
*EUR region sample sizes: Great Britain and Ireland, n = 165; Scotland and Ireland, n = 148; Scandinavia, n = 59; Midcontinental EUR, n = 76; EUR except Great Britain and Ireland, n = 135.
Shipping Records.
We documented 882 European ships entering Pictou Harbor from 1773 to 1861 (Table S3). This period was studied because (i) Pictou's first immigrants from Europe arrived in 1773 on the Hector and began a lucrative timber trade back to Great Britain (22, 23) and (ii) both F. serratus and L. littorea must have been present by at least 1861 [F. serratus appears to have been common in Pictou Harbor in 1868 (based on Rev. Fowler's herbarium sheet in Farlow Herbarium, Harvard University)]. Shipping records were grouped (1773–1815, 1816–1827, 1828–1845, and 1846–1861) because of historical events that shaped shipping patterns (Table 3).
Table 3.
European ships entering Pictou Harbor, Nova Scotia, 1773–1861
| Period | 1773–1815* | 1816–1827† | 1828–1845‡ | 1846–1861§ | Totals |
|---|---|---|---|---|---|
| Key events | Arrival of Hector in Pictou (1773) with first of many Scottish immigrants. Founding of large timber trade (Pictou–Britain) due to the Napoleonic Wars (22, 23) | Famine and depression at end (1815) of Napoleonic Wars increase Celtic emigration to Nova Scotia, including Cape Breton (24, 27) | Pictou becomes a Free Port, open to non-British ships; timber trade declines (22). Famine in Scotland (1836–37). L. littorea abundant in Pictou (12) | Great Irish potato famine begins (1845). F. serratus found (1868) in Pictou | — |
| Total ships (n) | 89 | 236 | 404 | 153 | 882 |
| English, % | 3.4 | 32.2 | 61.1 | 41.8 | 44.2 |
| Irish, % | 0 | 5.5 | 5.4 | 10.5 | 5.8 |
| Scottish, % | 96.6 | 61.9 | 28.5 | 45.8 | 47.3 |
| Continental Europe, % | 0 | 0 | 4.7 | 1.3 | 2.4 |
| Total ports | 26* | 37† | 51‡ | 24§ | 79 |
Major outbound ports/period (%, total ships) provided in footnotes (Table S3 lists individual ships, Dataset S2 lists all ports/period).
*Scotland: Aberdeen (18.0%), Greenock/Port Glasgow (25.8%), Stornoway/Ullapool (11.2%).
†England: Liverpool (17.8%), Newcastle/Shields (6.8%); Scotland: Aberdeen (24.6%), Greenock/Port Glasgow (13.6%), Cromarty/Inverness (11.2%), Leith/Firth of Forth (7.2%).
‡England: Hull (5.9%), Liverpool (17.3%), London (6.9%), Newcastle/Shields (14.1%). Scotland: Cromarty/Inverness (5.9%), Greenock/Glasgow (14.1%).
§England: Liverpool (22.2%); Scotland: Glasgow/Greenock (43.8%).
More ships sailed to Pictou from Scotland (47.3%, Table 3) than elsewhere, especially in the first 2 periods (96.6% and 61.9%) when Scottish immigration was highest. English shipping increased during the last 2 periods (61.1%, 41.8%, Table 3). Only ships from Great Britain and Ireland sailed to Pictou until 1828, when Pictou and Sydney (Fig. 1) joined Halifax as Free Ports (Table 3, Table S3, Dataset S2, and ref. 22). Although Free Port status allowed vessels from France, Germany, Belgium, The Netherlands, and the Baltic to arrive after 1828, only 2.4% of the 882 European ships arriving in Pictou came from outside Great Britain and Ireland. During 1846–1861, the number of European arrivals was halved (10.2 ships per year) relative to the preceding 2 periods. The proportion of entries from continental Europe remained small, and the proportion of Scottish (45.8%) and Irish (10.5%) ships increased (Table 3).
Ships sailed between 1773 and 1845 to Pictou from 39 Scottish ports and 21 English ports (Dataset S2), but some ports were more important than others (Table 3, i.e., Scotland: Aberdeen, Cromarty, Greenock, Glasgow; England: Liverpool, London, Newcastle). Irish ports were best represented between 1845 and 1861 because of Great Famine-related emigration (Table 3, Table S3, and Dataset S2). Notably, no ship arrived in Pictou from Galway, Ireland, but ships did sail to Pictou from western and southern Ireland (e.g., Sligo, Limerick, Cork; see Table S3, and Dataset S2). The timber trade and Scottish settlement expanded to Cape Breton Island (CBI, Fig. 1) by the early 1800s (23); although customs/shipping records for CBI ports began later than Pictou, the source patterns are similar (e.g., refs. 23 and 24).
Estimated Times of Introduction.
F. serratus lacks a long-lived dispersal stage and spreads slowly after colonization (25). We estimate that it arrived in Pictou sometime between 1824 and 1858 [i.e., before 1868] based on estimates of colonization rates in Nova Scotia and the distance (15 km) between Pictou Harbor and Pictou Island, to which it had spread by 1887 (13). Direct estimates of colonization rates of 0.24–0.52 km/y were obtained by comparing earlier (7, 26) to more recent distributions in Nova Scotia; these rates were similar to rates measured for introduced F. serratus in Iceland (0.3–0.6 km/y) (18).
The estimated timing of L. littorea introduction was determined indirectly by a coalescence analysis of divergence times using the isolation with migration (IMa) program (Table S4). Using a range of cytochrome b mutation rates (2–4% MY), we calculated periods of: (i) 192-11,794 years BP (Nova Scotian haplotypes vs. haplotypes from Great Britain/Ireland), (ii) 2,555-20,210 years BP (Nova Scotian haplotypes vs. all European haplotypes), and (iii) 10,763-82,995 years BP (Nova Scotian haplotypes vs. all European haplotypes except haplotypes from Great Britain/Ireland). Comparisons using just haplotypes from Great Britain/Ireland resulted in divergence estimates (Table S4) that more closely matched historical reports of Nova Scotian L. littorea, whereas inclusion of haplotypes from continental Europe (especially Scandinavia) greatly inflated divergence estimates.
Discussion
Great Britain and Ireland are the most probable source regions for F. serratus and L. littorea. For F. serratus, the power of our analysis is high, given our extensive sampling, and the species' slow dispersal rate and microsatellite allelic diversity. Such biological features result in a high degree of genetic structure in populations on both sides of the Atlantic, which allowed identification of source locations in Europe on the order of 50–100 km. This high level of spatial precision cannot be achieved for L. littorea, because its widely dispersing planktonic larvae result in extensive gene flow over hundreds of kilometers. Indeed, given the intervening >170 y between its appearance in Nova Scotia and our study, it is encouraging that we were able to determine the species' source region to be Great Britain and Ireland.
Rock ballast was almost certainly the vector of these introductions. The traditional supply of Baltic lumber to Great Britain was greatly reduced by the Napoleonic Wars, which fostered the development of the Nova Scotian timber trade (22, 23). Many ballasted ships arrived in Pictou carrying passengers or goods and then discharged ballast before returning to Great Britain with heavy loads of timber. The Pictou timber trade began in 1774 and spread to western CBI by the early 1800s, where ballast was also discharged (23). The prosperous lumber trade attracted many Scottish emigrants because of economic depression and famine in Scotland after the Napoleonic Wars (1815) (27). These facts account for the many ports (putative invasion sources) involved in early Scottish immigration to Nova Scotia.
Ballast discharge became a concern in Pictou because of the careless manner in which ships discharged ballast. In 1821, local magistrates demarcated an area of the shallow harbor where ships were required to discharge ballast to keep the rest of the harbor clear (28, 29). Later, ballast was again discharged on the shore (22). Some ships acquired their ballast from the intertidal zone (e.g., 30), essentially transporting parts of these intertidal communities from Great Britain/Ireland to North America. An intentional introduction of L. littorea for food (31) cannot be completely dismissed; however, our finding of multiple introductions of F. serratus into Pictou and western CBI highlights the ballast pathway and sufficiently explains the introduction of L. littorea, which may also have occurred multiple times at multiple sites, including with F. serratus.
The invasion by L. littorea must have occurred earlier than the first recorded discovery, because it was “abundant” at Pictou by 1841 (12) and found throughout eastern Nova Scotia by the early 1860s [possibly by the early 1800s (32)]. Our coalescence simulations of L. littorea with expanded sampling of Irish and Scottish ports bring the molecular estimate of the introduction within this time period (although both younger and older dates are within the range). Considering all of the evidence, the introduction of L. littorea into Nova Scotia probably occurred in the late 18th/early 19th Century, when shipping was dominated by Scottish vessels in eastern Nova Scotia (Table 3) and by Scottish and English vessels at Halifax (23, 24, 33). Midcontinental Europe had the second highest number of haplotypes shared with Pictou (or Nova Scotia) after Great Britain/Ireland, probably because of the close proximity of influential sites in Brittany to the glacial refugium (LGM) in the western English Channel, from which recolonization into Great Britain/Ireland occurred (e.g., ref. 11). Continental European shipping did not provide propagule pressure for the introductions of F. serratus and L. littorea because French influence in the Canadian Maritimes diminished in 1713 (Treaty of Utrecht) and ended in 1763 (Peace of Paris). Further, we documented only a few ships sailing from continental Europe to Pictou after it became a Free Port (1828). Thus, propagule pressure for introduction of both L. littorea and F. serratus from Great Britain and Ireland was especially high.
An unresolved question concerns the rare subfossils of L. littorea (500–1,500 y BP) in a few shore deposits and middens in the Canadian Maritimes and the 33,000 y BP Nova Scotian fossil (reviewed by refs. 15 and 16). L. littorea is dioecious, reproduces by copulation, and spawns fertilized eggs seasonally in the NW Atlantic (34). Given the haplotype matches to the European region of highest shipping traffic when L. littorea first became abundant, we hypothesize that these rare fossils are representative of occasional introductions [e.g., on driftwood or floating seaweed (15)], which failed to produce sufficient contemporaneous numbers of individuals for effective reproduction and invasion of North America.
The actual number of viable propagules released by each potential introduction is rarely known (35). However, proxies can provide an estimate (36, 37), and we used the number of ships connecting each European port to Pictou Harbor as such a proxy. At the coarsest scale, ≈98% of the ships sailing to Pictou (similar patterns for western CBI) were from Great Britain and Ireland and ≈2% from continental Europe (Table 3). By using the shipping records alone, Scottish, English, and last, Irish ports would be identified as the most likely sources. The strength of Scottish propagule pressure appears in our precise assignment of the F. serratus introduction in western CBI to Greenock, Scotland. However, for Pictou, the source was clearly Galway, Ireland, even though Irish ports accounted for only 5.8% of all shipping traffic (Table 3), and no ships are recorded arriving in Pictou from Galway (ref. 22; Table S3, and Dataset S2). Although this result challenges the importance of propagule pressure, recycling (38) of ballast (bearing F. serratus) from Galway ships to at least 1 Pictou-bound ship could have occurred in Great Britain/Ireland (N.B., we have not found a 3-point voyage by a single ship). Preliminary investigation of Galway shipping (n > 1,000 departing ships, Galway Vindicator, 1841–1854) shows that the timing of intracontinental Galway arrivals in Glasgow, Greenock, Limerick, Liverpool, London, and Newcastle (Table S5) offered opportunities for ballast to be recycled from a Galway ship to one bound for Pictou from those ports. Additionally, some ships sailed to British North America after intermediate stops at Irish ports (e.g., ref. 38) for supplies and repair (39); although no intermediate Galway stops enroute to Pictou are recorded, they may have occurred. As discussed, the case for L. littorea is less precise because of the species' biology and high haplotype diversity; however, our molecular analyses were corroborated by the Pictou shipping records in which 98% of ships sailing to Pictou originated from Great Britain and Ireland, and our molecular data most closely matched haplotypes in Great Britain and Ireland.
Why did these introductions, at least those of F. serratus, occur in the Gulf of St. Lawrence area of Nova Scotia (i.e., Pictou, western CBI) and not in Atlantic ports of British North America, many of which also received ballast from Great Britain and Ireland? One possibility is that disturbance regimes may have played a role in these invasions, as they do in many modern ones (40). For example, an experimental study in SW England (41) showed that F. serratus had poor invasibility into functional groups (e.g., canopy and turf) that are more common on Atlantic shores than on Gulf shores because of the friable rock (e.g., sandstone, slate) and strong ice scour of Gulf shores (Pictou shipping begins in late April and ends in December because of blockage of the Gulf by sea ice, Table S3). Additionally, the significant amounts of rock ballast dumped underwater created hard substratum below the winter ice pack; this may have facilitated successful colonization by F. serratus on newly dumped ballast.
We have demonstrated the value of a multidisciplinary approach for examining biological invasions. By combining historical, genetic, and ecological data, we refined understanding of 2 particular introductions while exploring limits of resolution for 4 fundamental questions in invasion biology: Where did the invader first become established? When did it arrive? Where did it come from? How did it get there? Beyond these immediate results, our data contribute to a broader understanding of marine communities. First, F. serratus and L. littorea must be the tip of an “invasion iceberg” involving a suite of associated, but less conspicuous, species that followed the same path from Great Britain and Ireland to North America through Nova Scotia. Communities on both sides of the Atlantic should now be examined for other invaders that have been considered indigenous or at best “cryptogenic” (17, 42, 43), with better ability to assess the role of source-specific genetic adaptation to invasion success (44). Second, all introductions must be evaluated against relevant ecological and evolutionary processes (40, 45) and integrated into our understanding of community structure and function (31), especially in terms of long-term changes and our perception of what is natural (46) or desirable in our marine environment. It can be difficult to compare recipient and source habitats directly because many port areas are completely transformed from their 19th Century states. Nevertheless, adjacent areas can provide clues about habitat, ecotypic identity, and diversity that underlie our interest in eventual comparisons of community structure on northern Atlantic rocky coasts. Indeed, the emerging concern of “sliding baselines” in the assessment of our natural world (47, 48) requires that we know what came from where and when.
Materials and Methods
(see SI Text and Table S6 for details). Shipping records were collected from primary sources [e.g., newspapers, customs' documents (33) in Canada, Scotland, and Ireland, as well as from secondary sources (22–23, 24); SI Text]. Nova Scotian populations of F. serratus [Pictou, Inverness (CBI), Caplin Cove (CBI)] were compared with European populations with 7 microsatellite primers following previous protocols (49, 50). SI Text and Table S6 provide information on analyses with GENETIX 4.02, GENCLONE 1.0 (β version), and GENECLASS2.
DNA extractions of L. littorea and genetic analyses based on cytochrome b haplotypes followed published protocols (9). Previously unreported haplotypes have GenBank accession nos. FJ750983–FJ751157. F. serratus distribution was updated by surveying the Canadian shore between 2005 and 2007; this permitted calculation of migration rates to estimate time of introduction at Pictou. Population divergence estimates for Nova Scotian L. littorea were compared with European populations by using IMa as described (SI Text, and Table S6).
Supplementary Material
Acknowledgments.
We thank J. Hey, C. Cunningham, J. Carlton, and M. Guiry for discussion. S.H.B. thanks archivists at Public Archives of Nova Scotia (Philip Hartley), Hector Archive (Darrell Burke), National Archives of Scotland, National University of Ireland, Galway, Ireland, and Galway County libraries, and the University of Maine (Susan Buzzell and Barbara Jones); the DNA Sequencing Center (University of Maine); Michael Guiry (Galway), David Mann (Edinburgh), Suzanne Craig and Daniel Rochaix (Mabou, Nova Scotia) for hospitality. The National Science Foundation (NSF) Research Coordination Networks–CORONA (C. Cunningham, principal investigator) aided in project development. J.L.O., J.A.C. and G.H. thank Jan Veldsink for additional genotyping of F. serratus. This work was supported by National Geographic Society Grant 7827-05 (to S.H.B. and L.E.J.), NSF Grants OCE 0622439 (to S.H.B.) and OCE 0503932 (to J.E.B.), the Canadian–American Center (University of Maine, S.H.B.), and The Alfred P. Sloan Foundation (HMAP funding, A.M.H.B.; A. Rosenberg, principal investigator).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Cytochrome b sequences for L. littorea have been deposited in the GenBank database (accession nos. FJ750983–FJ751157).
This article contains supporting information online at www.pnas.org/cgi/content/full/0812300106/DCSupplemental.
References
- 1.Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH. Invasion of coastal marine communities in North America: Apparent patterns, processes, and biases. Annu Rev Ecol Syst. 2000;31:481–531. [Google Scholar]
- 2.Molnar JL, Gamboa RL, Revenga C, Spalding MD. Assessing the global threat of invasive species to marine biodiversity. Front Ecol Env. 2008;6:485–492. [Google Scholar]
- 3.Kolar CS, Lodge DM. Progress in invasion biology: Predicting invaders. Trends Ecol Evol. 2001;16:199–204. doi: 10.1016/s0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- 4.Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol. 2005;20:223–228. doi: 10.1016/j.tree.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Colautti RI, Grigorovich IA, MacIsaac HJ. Propagule pressure: A null model for biological invasions. Biol Inv. 2006;8:1023–1037. [Google Scholar]
- 6.Reaser JK, Meyerson LA, Von Holle B. Saving camels from straws: how propagule pressure-based prevention policies can reduce the risk of biological invasion. Biol Inv. 2008;10:1085–1098. [Google Scholar]
- 7.Edelstein T, Greenwell M, Bird CJ, McLachlan J. Investigations of the marine algae of Nova Scotia. X. Distribution of Fucus serratus L. and some other species of Fucus in the Maritime Provinces. Proc N S Inst Sci. 1971–73;27:33–42. [Google Scholar]
- 8.Vadas RL, Sr, Elner RW. In: Plant-Animal Interactions in the Marine Benthos. John DM, Hawkins SJ, Price JH, editors. Oxford: Clarendon Press; 1992. pp. 33–60. [Google Scholar]
- 9.Blakeslee AMH, Byers JE, Lesser MP. Solving cryptogenic histories using host and parasite molecular genetics: The resolution of Littorina littorea's North American origin. Mol Ecol. 2008;17:3684–3696. doi: 10.1111/j.1365-294X.2008.03865.x. [DOI] [PubMed] [Google Scholar]
- 10.Coyer JA, Peters AF, Stam WT, Olsen JL. Post-Ice Age recolonization and differentiation of Fucus serratus L. (Fucaceae: Phaeophyta) populations in Northern Europe. Mol Ecol. 2003;12:1817–1829. doi: 10.1046/j.1365-294x.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoarau G, Coyer JA, Veldsink J, Stam WT, Olsen JL. Glacial refugia and recolonization pathways in the brown seaweed Fucus serratus. Mol Ecol. 2007a;16:3606–3616. doi: 10.1111/j.1365-294X.2007.03408.x. [DOI] [PubMed] [Google Scholar]
- 12.Dawson JW, Harrington BJ. Report on the Geological Structure of Prince Edward Island. Montreal, Canada: Government of Prince Edward Island; 1871. Appendix and Plates. [Google Scholar]
- 13.Hay GU, MacKay AH. Marine algae of New Brunswick. Trans R Soc Canada. 1887;5:167–174. [Google Scholar]
- 14.Ganong WF. Is Littorina littorea introduced or indigenous? Am Nat. 1887;21:287–288. [Google Scholar]
- 15.Reid DG. Systematics and Evolution of Littorina. Dorchester, UK: Dorset; 1996. [Google Scholar]
- 16.Leifsdóttir ÓE, Símonarson LA. The mesogastropod Littorina littorea (Linné, 1758) in Iceland: Palaeobiology and migration. Cainozoic Res. 2002;1:3–12. [Google Scholar]
- 17.Blakeslee AMH, Byers JE. Using parasites to inform ecological history: Comparisons among three congeneric marine snails in North America and Europe. Ecology. 2008;89:1068–1078. doi: 10.1890/07-0832.1. [DOI] [PubMed] [Google Scholar]
- 18.Coyer JA, Hoarau G, Skage M, Stam WT, Olsen JL. Origin of Fucus serratus (Heterokontophyta; Fucaceae) populations in Iceland and the Faroes: A microsatellite-based assignment. Eur J Phycol. 2006;41:235–246. [Google Scholar]
- 19.Robinson CB. The distribution of Fucus serratus in North America. Torreya. 1903;3:132–134. [Google Scholar]
- 20.Rannala B, Mountain JL. Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA. 1997;94:9197–9201. doi: 10.1073/pnas.94.17.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colwell RK. EstimateS: Statistical estimation of species richness and shared species from samples. [Accessed April 20, 2009];2005 Version 7.5. Available at http://purl.oclc.org/estimates.
- 22.Patterson G. A History of the County of Pictou, Nova Scotia. Montreal: Dawson; 1877. [Google Scholar]
- 23.Campey LH. After the Hector. The Scottish Pioneers of Nova Scotia and Cape Breton 1773–1852. Toronto: Natural Heritage Books; 2004. [Google Scholar]
- 24.Martell JS. Immigration to and Emigration from Nova Scotia 1815–1838. Halifax: The Public Archives of Nova Scotia; 1942. [Google Scholar]
- 25.Pearson GA, Serrão EA. Revisiting synchronous gamete release by fucoid algae in the intertidal zone: Fertilization success and beyond? Integr Comp Biol. 2006;46:587–597. doi: 10.1093/icb/icl030. [DOI] [PubMed] [Google Scholar]
- 26.MacFarlane C. A survey of certain seaweeds of commercial importance in southwest Nova Scotia. Can J Bot. 1952;30:78–97. [Google Scholar]
- 27.Select Committee on Emigration, Scotland. First Report, Minutes of Evidence. London: House of Commons; 1841. accessed via House of Commons Parliamentary Papers Online, ProQuest Information and Learning Company. [Google Scholar]
- 28.Anonymous. The Statutes at Large Passed in the Several General Assemblies. Halifax: Legislature; 1919. An Act to Preserve and Regulate the Navigation of the Harbour of Pictou. held as J104 H6 1817–1826 in Public Archives of Nova Scotia, Halifax. [Google Scholar]
- 29.Pictou Sessional Book. 1820;Vol 318:5. entry: February 15, 1820 (held at Public Archives of Nova Scotia, Halifax as RG-34) [Google Scholar]
- 30.Bigger W. London: House of Commons Parliamentary Papers; 1830. Ballast Office Corporation, Dublin (14 April 1830) accessed via House of Commons Parliamentary Papers Online. [Google Scholar]
- 31.Steneck RS, Carlton JT. In: Marine Community Ecology. Bertness M, Gaines S, Hay M, editors. Sunderland, MA: Sinauer; 2000. pp. 445–468. [Google Scholar]
- 32.Willis JR. On the occurrence of Littorina Littorea on the coast of Nova Scotia. Proc Trans NS Inst Nat Sci. 1863;1:88–90. [Google Scholar]
- 33.Public Archives of Nova Scotia. Halifax: 1814–1861. Documents of Collector of Colonial Duties (RG 31–102) and Impost and Excise (RG31-104) for Nova Scotia. [Google Scholar]
- 34.Chase ME, Thomas MLH. The effect of the rate and onset of temperature increase on spawning of the periwinkle, Littorina littorea (L) J Exp Mar Biol Ecol. 1995;186:277–287. [Google Scholar]
- 35.Cassey P, et al. Global patterns of introduction effort and establishment success in birds. Proc R Soc London Ser B. 2004;271(Suppl 6):S405–S408. doi: 10.1098/rsbl.2004.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung B, Drake JM, Lodge DM. Predicting invasions: Propagule pressure and the gravity of allee effects. Ecology. 2004;85:1651–1660. [Google Scholar]
- 37.Wonham MJ, Carlton JT. Trends in marine biological invasions at local and regional scales: The Northeast Pacific Ocean as a model system. Biol Inv. 2005;7:369–392. [Google Scholar]
- 38.Lindroth CH. The Faunal Connections Between Europe and North America. New York: Wiley; 1957. [Google Scholar]
- 39.Galway Vindicator. Muzurke. Glasgow to Montreal; 1852. Port News, 5-15-1852. [Google Scholar]
- 40.Sax DF, Stachowicz JJ, Gaines SD. Species Invasions: Insights into Ecology, Evolution and Biogeography. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- 41.Arenas F, Sánchez I, Hawkins S, Jenkins SR. The invasibility of marine algal assemblages: Role of functional diversity and identity. Ecology. 2006;87:2851–2861. doi: 10.1890/0012-9658(2006)87[2851:tiomaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.van Oppen MJH, Draisma GA, Olsen JL, Stam WT. Multiple trans-Arctic passages in the red alga Phycodrys rubens: Evidence from nuclear rDNA ITS sequences. Mar Biol. 1995;123:179–188. [Google Scholar]
- 43.Carlton JT. Biological invasions and cryptogenic species. Ecology. 1996;77:1653–1655. [Google Scholar]
- 44.Roman J. Diluting the founder effect: Cryptic invasions expand a marine invader's range. Proc R Soc Ser B. 2006;273:2453–2459. doi: 10.1098/rspb.2006.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sax DF, et al. Ecological and evolutionary insights from species invasions. Trends Ecol Evol. 2007;22:465–471. doi: 10.1016/j.tree.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 47.Pauly D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol. 1995;10:430. doi: 10.1016/s0169-5347(00)89171-5. [DOI] [PubMed] [Google Scholar]
- 48.Knowlton N, Jackson JBC. Shifting baselines, local impacts, and global change on coral reefs. PLOS. 2008;6:215–220. doi: 10.1371/journal.pbio.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coyer JA, Veldsink JH, Stam WT, Olsen JL. Characterization of microsatellite loci in the marine rockweeds, Fucus serratus and F evanescens (Heterokontophyta; Fucaceae) Mol Ecol Notes. 2002;2:35–37. [Google Scholar]
- 50.Hoarau G, Coyer JA, Stam WT, Olsen JL. A fast and inexpensive DNA extraction/purification method for brown macroalgae. Mol Ecol Notes. 2007b;7:191–193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.