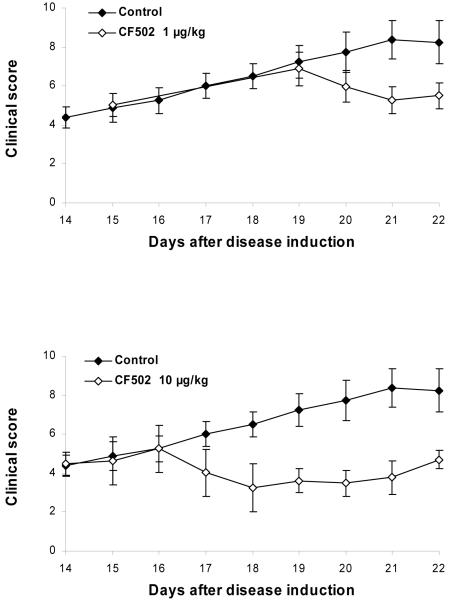
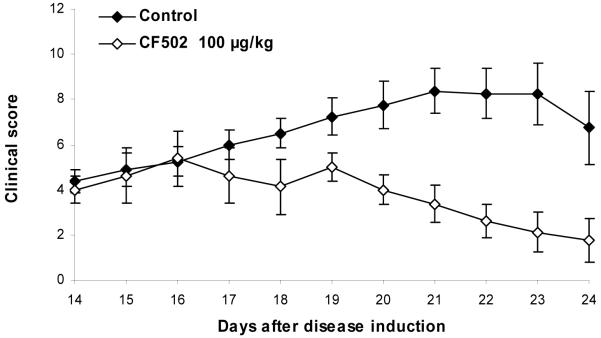
Figure 5.
Effect of CF502 at various dosages on the clinical and pathological manifestations of AIA. Rats were immunized with a single injection of Mycobacterium tuberculosis in incomplete Freund’s adjuvant. Treatment with CF502 was initiated upon the onset of arthritic disease. CF502 was administered orally, thrice daily. Clinical score of the arthritic disease during course of the studies was evaluated A significant dose-dependent decrease in the severity of the disease was noted. with a maximal inhibition at the dose of 100 μg/kg of CF502 (p< 0.05).