Abstract
This paper develops theory and methods for vaccine trials that utilize spatial and environmental information. Satellite imagery is used to identify whether households are connected to one another via water bodies in a study area in rural Bangladesh. Then relationships between neighborhood-level cholera vaccine coverage and placebo incidence and neighborhood-level spatial variables are measured. The study hypothesis is that unvaccinated people who are environmentally connected to people who have been vaccinated will be at lower risk compared to unvaccinated people who are environmentally connected to people who have not been vaccinated. We use four data sets including: a cholera vaccine trial database, a longitudinal demographic database of the rural population from which the vaccine trial participants were selected, a household-level geographic information system (GIS) database of the same study area, and high resolution Quickbird satellite imagery. An environmental connectivity metric was constructed by integrating the satellite imagery with the vaccine and demographic databases linked with GIS. The results show that there is a relationship between neighborhood rates of cholera vaccination and placebo incidence. Thus, people are indirectly protected when more people in their environmentally-connected neighborhood are vaccinated. This result is similar to our previous work that used a simpler Euclidean distance neighborhood to measure neighborhood vaccine coverage (Ali et al., 2005). Our new method of measuring environmental connectivity is more precise since it takes into account the transmission mode of cholera and therefore this study validates our assertion that the oral cholera vaccine provides indirect protection in addition to direct protection.
Keywords: Bangladesh, spatial analysis, vaccine trials, cholera, satellite imagery
1. Introduction
This paper develops new theory and methods for geographic vaccine trials. Conventional vaccine trial methods have an underlying assumption that the effect of the vaccine is the same throughout the trial area. Previously, we conducted a study to test whether this assumption is true for one vaccine trial using a spatially referenced database (Ali et al., 2005, 2008; Emch et al., 2006, 2007). Our results illustrated that the protective efficacy of oral cholera vaccines varies in space (Emch et al., 2007) and that the variation is inversely related to vaccine coverage (i.e., % of people vaccinated in an area) after adjusting for several ecological factors (Emch et al., 2006). We also found that higher levels of neighborhood vaccine coverage are linked to lower risk of cholera among residents (Ali et al., 2005, 2008). These findings show that higher levels of vaccine coverage can lead to higher levels of indirect protection of non-vaccinees, and may also lead to higher levels of total protection, i.e., indirect protection combined with direct protection of vaccines (Ali et al., 2005). We coined the term “ecological vaccine trials” because our approach includes neighborhood-level variables in addition to conventional individual-level variables commonly used in vaccine evaluation.
This paper extends the theory and methods we created for ecological vaccine trials developed in previous research by not only defining neighborhoods by spatial proximity using a geographic information system (GIS) as we did before, but also by using satellite data to better model the spatial variation of the environmental context in which people live. The theoretical consideration behind the spatial and environmental connectivity analysis in vaccine trials stemmed from an understanding that the level of vaccination is spatially variable (Bolker and Grenfell, 1996), and results observed in our previous work (Ali et al., 2005; Emch et al., 2006, 2007). The variation in the level of vaccination across space allows one to evaluate the intrinsic effect of neighborhood-level vaccine coverage on spatial dynamics of the target disease of an area. This is done by using high resolution satellite imagery to identify whether households are connected to one another via ponds. This is appropriate because cholera is a waterborne disease and when households are connected via water bodies it is more likely that the disease will spread to neighbors (Codeço, 2001). Conventional vaccine evaluation may be biased because of spatial variation in neighborhood-level vaccine coverage and different community-level characteristics. While our original geographic analysis of the vaccine data provided profound new insights into the incorporation of spatial theory and methods into vaccine trials, further analysis of these data is necessary because prior research used very simplistic measures to model space. Our initial model assumed that all people are just as likely to come into contact with one another within a given Euclidean neighborhood size. There are, however, many processes that modify distance. This study extends previous research by examining one such process, environmental connectivity. This paper thus considers the possibility that neighborhoods are not homogeneous and that some neighborhoods are more connected than others because of environmental (i.e., shared water bodies) connections.
We use four data sets including: (1) a cholera vaccine trial database, (2) a longitudinal demographic database of the rural population from which the vaccine trial participants were selected, (3) a household-level spatial database of the same study area, and (4) high resolution satellite imagery (Quickbird). These databases provide a unique opportunity to develop and test new vaccine trial methods. The cholera vaccine trial is one of the largest in history with approximately 49,336 two or three dose vaccinees, and the longitudinal demographic database of the study area is known to be the most comprehensive in the developing world. All vital demographic events in a population of approximately 200,000 people were noted every two weeks through an extensive community-based data collection system. A corresponding household-level GIS database allows us to identify the household location of all individuals who took part in the trial as well as the household location of each person in the demographic surveillance system (i.e., the background population). This spatial database, in conjunction with the demographic and vaccine datasets, facilitates adding an integrated, comprehensive, and accurate spatial component to all of the datasets. The datasets are also contemporaneous; the demographic and vaccine datasets were collected during the same time period, and the GIS database of households was created later but the locations of the houses did not change. While the Quickbird satellite images were collected after the study period (because the satellite had not yet been launched), we assume that the physical locations of the water bodies did not change very much.
The main study objective is to develop theory and methods that utilize spatial and environmental information that can be used in future vaccine trials. This is accomplished by measuring relationships between neighborhood vaccinee and placebo incidence and neighborhood-level spatial variables. Our research hypothesis is that placebo group incidence is influenced by environmental connectivity of households because herd protection is affected by how environmentally connected people are to one another. In other words, unvaccinated people who are environmentally connected to people who have been vaccinated will be at lower risk and unvaccinated people who are environmentally connected to people who have not been vaccinated will be at higher risk.
2. BACKGROUND AND DATA
In 1985, a community-based individually-randomized oral cholera vaccine trial was conducted in Matlab, Bangladesh, the research site for the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR, B). This double-blind trial measured the efficacy of two vaccines, the B subunit-killed whole cell (BS-WC) and the killed whole cell only (WC). The control agent was Escherichia coli K12 strain. Females aged 15 years and older and children aged 2 to 15 were the target group. Three vaccine doses were given in six-week intervals. The vaccine trial used a passive surveillance system to identify cholera cases from the study area. The surveillance took place at one hospital and two community-based treatment centers. During 5 years of follow-up, the protective efficacy was 49% for the BS-WC group (P<0.001) and 47% for the WC group (P<0.001). Protection was lower in children who were vaccinated at 2 to 5 years than in older persons. For children in this age group, protection waned after 4 to 6 months and was not evident at all during the third year. Vaccinated persons older than 5 years of age were protected even in the third year of follow-up (Clemens et al., 1990a).
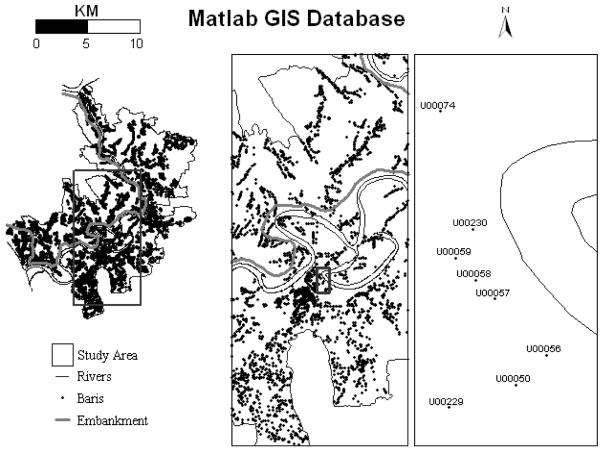
The research site for the ICDDR, B and for this project is called Matlab because the Centre’s hospital is located in Matlab Town. Matlab is in south-central Bangladesh, approximately 50 kilometers southeast of Dhaka, adjacent to where the Ganges River meets the Meghna River forming the Lower Meghna River. A demographic surveillance system (DSS) has recorded all vital events of the study area population since 1966; the study area population has been approximately 200,000 since that time. The database is the most comprehensive longitudinal demographic database of a large population in the developing world. The residents of the study area live in clusters of patrilineally-related groups of households called baris. We created a GIS database of the Matlab field research area (Emch, 1999; Ali et al., 2001). Features in digital format include baris, rivers, and health facilities. Figure 1 shows the GIS database at three different scales. The map view on the far right has the individual bari identification numbers visible. The baris are all identified by an ICDDR, B DSS census number, a unique number assigned to all individuals in the study area, within the structure of the GIS database. In turn, demographic data, disease incidence, or vaccine status data can be linked to specific bari locations. The Matlab field research center has in- and out-patient services, a medical laboratory, and research facilities. One-hundred twenty community health workers visit each household area every two weeks to collect demographic, morbidity, and other data.
Figure 1.
Study area GIS database
This study uses retrospective vaccine trial data collected in Matlab from 1985 to 1990 (Clemens et al., 1986a, 1986b, 1987, 1988a, 1988b, 1988c, 1988d, 1989a, 1989b, 1989c, 1990a, 1990b, 1991, 1992; Durham et al., 1998; Sack et al., 1991; van Loon et al., 1996). The objective of this randomized double-blind, placebo controlled trial was to determine whether three doses of BS-WC and WC vaccines reduces the incidence of laboratory confirmed cholera in 2 to 5 year old children and females over 15. The target group was individually randomized based on a simple random sampling scheme derived from DSS records. The Matlab GIS database includes an accurate bari location for all individuals living in the study area including all vaccinees, controls, people who refused vaccines, and everyone else living in the study area who was not part of the study. The GIS database also includes the locations of the treatment facilities that were used in the passive surveillance system for the vaccine trial. We have linked the individual level data from the vaccine trial to bari locations via the ICDDR, B DSS census identification number. The GIS thus facilitates the identification of the dwelling locations of individuals who participated in the clinical trial, as well as the entire population distribution of the Matlab study area.
Remotely sensed (RS) data has increasingly been used in the health sciences to assist researchers in identifying environmental factors - such as rainfall, humidity, temperature or vegetation cover - that promote disease transmission, vector propagation and the emergence and maintenance of disease foci. Much of this work has been done on vector-borne diseases, such as malaria and schistosomiasis, because the primary vectors of the disease require specific environmental conditions in order to survive and these habitats can be characterized using RS data. Increased transmission of water-borne diseases that do not require a non-human host, such as cholera and typhoid, is associated with environmental factors such as flooding, extreme rainfall events and warmer water temperatures, which can also be detected using RS data. Recent studies of cholera show that data on climatic factors, such as sea surface temperatures, sea surface height, and the presence of aquatic plants, acquired using RS data are related to cholera incidence and seasonality (Lobitz et al., 2000; Emch et al, 2008). But the bacterium V. cholerae is also endemic in ponds throughout rural Bangladesh regardless of these climatic factors. Geographic data on the location of these water bodies, however, does not currently exist and one of the objectives of this study is to develop GIS layers that characterize the aquatic environment in our study area. To this end, we use RS data to pinpoint the location of ponds in Matlab.
3. METHODS
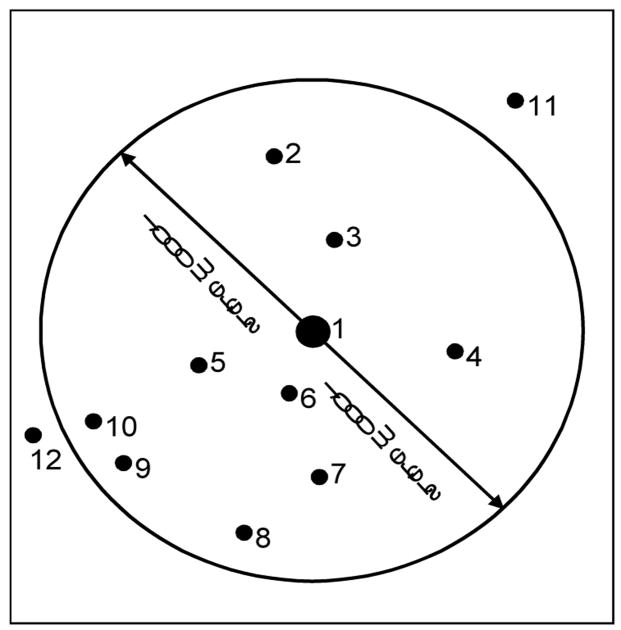
Traditionally, vaccine researchers have based trial evaluation only on individual characteristics (e.g., age and sex). Past vaccine trials have not stratified study data by location or environmental connectivity. In our previous work we calculated placebo incidence and vaccine efficacy based on Euclidean distance. For example, in Figure 2, there is a hypothetical 1 kilometer neighborhood around bari number 1 for which the placebo incidence, vaccinee incidence, and protective efficacy can be calculated. These calculations can only be made if population and disease data are available at the bari-level, and a GIS database that represents all baris as points is used to define spatial neighborhoods. The black dots in Figure 2 represent 12 different baris, and the circle represents a one kilometer neighborhood around bari number 1. There are 6,423 baris in the Matlab study area, thus the proportion of vaccinated people (i.e., coverage) can be computed for 6,423 neighborhoods centering on each bari. This Euclidean distance based neighborhood approach is the methodology employed in our previous work.
Figure 2.
One 1000-meter neighborhood
In this study we extend our previous research by examining how placebo and vaccine group incidence vary by vaccine coverage (i.e., proportion vaccinated) for people living in baris connected through the environment. First, using high resolution satellite imagery we developed metrics that measure environmental connectivity (e.g. whether baris are connected via water bodies). Second, we mapped vaccine coverage within local environmental neighborhoods. Third, we then measured relationships between placebo incidence and environmental neighborhood vaccine coverage while controlling for several possible confounders including: (a) age, (b) sex, (c) religion, (d) distance to nearest river, (e) distance to nearest treatment center, (e) and dysentery incidence.
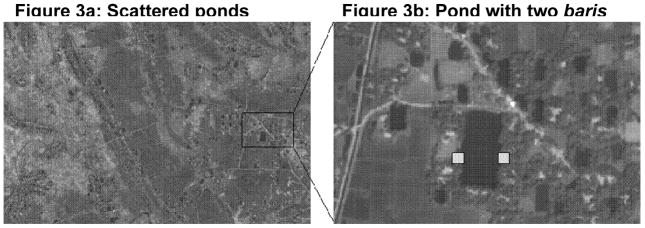
The first step in developing a measure of environmental connectivity was to integrate high resolution satellite imagery of the study area with the bari-level GIS database in order to characterize the aquatic environment in which V. cholerae bacteria live. Quickbird is the highest spatial resolution satellite imagery currently available (panchromatic band-61 centimeters, multispectral bands-4 meters). Such a high resolution is necessary to accurately locate small landscape features such as ponds. Figure 3a shows part of the study area with many ponds and Figure 3b is a magnified area of 3a that shows one pond with two hypothetical baris (identified by the two light-grey squares). If these two baris are connected to one another via a pond then sick people in those households would be more likely to contaminate each other than another set of two households the same distance from each other but without a pond connecting them.
Figure 3.
Figure 3a. Scattered ponds
Figure 3b. Pond with two baris
Three Quickbird satellite images of the study area (captured April and May of 2002) were mosaicked and processed in order to clearly delineate all ponds. The following image enhancement methods were used. In Erdas Imagine software, both the visible and the near infrared multispectral bands were displayed in a false-color composite. A tassled cap transformation (Crist and Kauth, 1986) was also created to better differentiate between water and other features including vegetation and soil. These two layers were displayed in combination with the panchromatic band of the Quickbird imagery in order to simultaneously display the fine spatial resolution of the panchromatic band and the richer spectral information from the multispectral bands. This method clearly displays the ponds which can be differentiated from other water bodies such as flooded areas. The ponds were digitized using heads-up digitizing techniques by using these aforementioned image combinations and enhancements but also the human brain is extremely good at determining spatial form of surface features including ponds. We then integrated the ponds layer with the GIS database shown in Figure 1. Figure 4 shows a small area of the Matlab study area with the digitized ponds. The background shows the Quickbird image that has been enhanced using the tasseled cap transformation. The grey polygons represent the digitized ponds and the black dots represent baris. Once the different datasets were integrated within GIS software, we developed a matrix that shows which households are connected via water bodies. The environmental connectivity metric was developed by creating a file showing links between each bari and all water bodies within 500 meters of the bari (e.g. the “environmental neighborhood”). We chose a 500 meter neighborhood size because we wanted our results to be comparable to our previous study (Ali et al., 2005), which also used a 500 meter neighborhood. Alternative distances were also examined (e.g., 1000 meters) in this study, but the 500 meter distance yielded the best results. We then computed the vaccine coverage rate for each bari based on connections to people living in the environmental neighborhood. We hypothesized that if cholera is transmitted through these water bodies and the vaccine exerts herd protection then higher levels of vaccine coverage in these environmental neighborhoods will have lower disease incidence rates for both vaccinated and unvaccinated population.
Figure 4.
Digitized ponds and baris overlaid with processed Quickbird imagery
In order to analyze the relationship between vaccine coverage and disease incidence, we built logistic regression models using generalized estimating equations with a logit link function to control for bari level clustering. The dependent variable unit was the individual and the level of vaccine coverage within an environmentally connected neighborhood was the main independent variable of interest. The models also controlled for potential confounding variables (age, sex, religion, distance to nearest river, distance to nearest treatment center, and dysentery incidence). Coefficients of independent variables in the models were exponentiated to estimate the odds ratio of cholera associated with different levels of coverage. Standard errors for the coefficients were used to estimate p values and associated 95% confidence intervals for the odds ratios.
4. RESULTS
Table 1 shows the risk of cholera among persons who received both the cholera vaccine and placebo by level of cholera vaccine coverage calculated through the environmental connectivity analysis during the first year of follow-up. The risk of cholera in recipients of two or three doses of either vaccine or placebo was inversely related to the level of vaccine coverage. This trend was significant in both placebo (p=<0·001) and vaccine recipients (p=0·03). The difference between the risk of cholera in the highest and lowest quintiles for environmental vaccine coverage was larger for placebo recipients than for vaccine recipients. Table 2 shows the relationship between vaccine coverage within environmental neighborhoods and an individual’s risk of cholera in models that used generalized estimating equations with the logit link function and that controlled for potential confounding variables known to be associated with the risk of cholera in the study area. In these models, we also controlled for whether an individual had experienced dysentery during follow-up to adjust for confounding effects not captured by the other variables in the models. Three separate models were built for (1) both vaccine and placebo recipients, (2) vaccine recipients only, and (3) placebo recipients only. In the combined model, vaccination of the individual and the level of vaccine coverage measured through environmental connectivity were each shown to have independent protective effects on cholera risk. Models 2 and 3 show that the inverse relation between cholera risk and the level of vaccine coverage was more pronounced for placebo recipients (p<.001) than for vaccine recipients (p=.05) while controlling for other known risk factors of cholera.
Table 1.
Cholera risk by level of cholera vaccine coverage measured through environmental connectivity.
| Level of vaccine coverage (%)‡ | Target population | Vaccinees | Placebo recipients | ||||
|---|---|---|---|---|---|---|---|
| N | Cases | Risk per 1,000 persons* | N | Cases | Risk per 1,000 persons† | ||
| ≤30.61 | 24,295 | 5,506 | 16 | 2.90 | 2,741 | 21 | 7.66 |
| 30.62–40.02 | 24,181 | 8,625 | 14 | 1.62 | 4,284 | 24 | 5.60 |
| 40.03–44.96 | 24,230 | 10,254 | 21 | 2.04 | 5,253 | 22 | 4.18 |
| 44.97–50.63 | 24,300 | 11,701 | 29 | 2.47 | 5,889 | 26 | 4.41 |
| 50.64+ | 24,143 | 13,250 | 16 | 1.20 | 6,500 | 15 | 2.30 |
| Total | 121,149 | 49,336 | 96 | 1.94 | 24,667 | 108 | 4.37 |
P=.03 for trend
P<.001 for trend
Baris are arranged by vaccine coverage into quintiles, each having approximately the same age- and gender-eligible population
Table 2.
Predictors of the cholera risk among vaccine and placebo recipients.
| Independent variables in the model | Model 1: All recipients of ≥2 doses (n=74,003) | Model 2: Recipients of ≥2 doses of vaccine (n=49,336) | Model 3: Recipients of ≥2 doses of placebo (n=24,667) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR* | P-value | 95% C.I. | R* | P-value | 95% C.I. | OR* | P-value | 95% C.I. | |
| Age (in years) | .98 | .001 | 0.96–0.99 | .95 | <.01 | 0.92–0.98 | 0.99 | .20 | 0.98–1.00 |
| Gender (female vs. male) | .14 | 43 | 0.83–1.56 | .18 | 45 | 0.77–1.80 | 1.05 | .81 | 0.68–1.62 |
| Religion (Hindu vs. non-Hindu) | .03 | .90 | 0.65–1.62 | .12 | .72 | 0.58–2.18 | 0.97 | .93 | 0.53–1.78 |
| Distance from bari to nearest river (kilometers) | .89 | .13 | 0.78–1.03 | .86 | .11 | 0.71–1.03 | 0.94 | .57 | 0.78–1.15 |
| Distance from bari to nearest treatment center (kilometers) | .13 | <.01 | 1.05–1.23 | .14 | .01 | 1.03–1.27 | 1.12 | .04 | 1.01–1.25 |
| Experienced dysentery during follow-up (yes/no) | .55 | .01 | 1.39–14.88 | .09 | .01 | 1.49–24.93 | 3.09 | .25 | 0.45–21.18 |
| Received ≥2 doses (vaccine vs. placebo) | .45 | <.001 | 0.34–0.59 | --† | -- | -- | --† | -- | -- |
| Level of cholera vaccine coverage of the bari (%) | .97 | <.001 | 0.96–0.99 | .98 | .05 | 0.96–1.00 | .97 | <.001 | 0.95–0.98 |
Multivariate odds ratio for the cited variable, adjusted for all other variables in the table, in a model using Generalized Estimating Equations (GEE) with the logit link function.
Variable for vaccination was not considered in models 2 and 3, since all subjects were either vaccinated or not vaccinated in these models.
5. DISCUSSION AND CONCLUSIONS
The results from this study show that when measuring vaccine coverage within environmental neighborhoods, people are indirectly protected when more people in their neighborhood are vaccinated. The results are very similar to our previous work that used a Euclidean distance neighborhood (Ali et al., 2005). In the Euclidean distance neighborhood used in our prior study, we observed 7.01 cases per 1,000 in clusters in the lowest quintile of coverage vs. 1.47 cases per 1,000 in clusters in the highest quintile, which were 7.66 and 2.30 respectively in the environmental connectivity-based neighborhoods used in this study. The trend of this relationship is statistically significant (p<0.01) in both cases. Our new method for measuring environmental neighborhoods is more precise since it takes into account the transmission mode of cholera. Since cholera is a waterborne disease, we believe that defining a neighborhood using connectivity of households through water bodies is more precise than using a general geographical proximity neighborhood. These results further validate our previous assertion that the oral cholera vaccine provides indirect protection above and beyond the direct effect of the vaccine. This study is a theoretical and methodological exercise that determined whether indirect effects can be measured using neighborhoods defined through environmental contact. Using high resolution satellite imagery and a spatially referenced vaccine database to measure environmental connections of recipients is a very time-consuming process. Since the results are similar when using the Euclidean distance neighborhood metric it begs the question, is this extra effort worth it? Possibly not in all vaccine trials, but a more precise neighborhood definition validates previous findings because it reflects the geographic reality of transmission better than a neighborhood definition purely based on spatial proximity.
The use of remotely sensed data and the techniques used to transform this data into vector data layers easily integrated with prior geographically referenced vaccine and demographic data in a GIS should be useful for researchers working in areas where little or no geographically referenced data of the aquatic environment is available. This was the case with Matlab and without the Quickbird imagery, we could not locate ponds, which are known to contain the V. cholerae bacterium, and would not have been able to develop the environmental neighborhoods. This technique could be extended to study other water-borne diseases or vector-borne diseases that require water for the vector to propagate.
There are several future directions that this exploration into geographic methods in vaccine trials will take. First, we will not limit the environmental connectivity metric to water bodies within 500 meter neighborhoods. The process of indirect protection is certainly scale dependent and understanding at what range of scales this process exists can help us understand how cholera is transmitted. We will also explore the use of more complex (continuous) environmental connectivity metrics that weight environmental ties by a distance decay function. This will involve creating a distance matrix between bari points and water bodies which can be easily done in GIS software (e.g., ArcGIS Pointdistance program). Furthermore, connections through the environment are not the only way to model connections between people that might lead to cholera transmission. Social network analysis can be used to measure relationships between social entities (Wasserman & Faust, 1994; Hanneman, 2001). They are particularly useful for measuring social relationships that influence disease outcomes or health interventions (Morris, 2004). Our future work will measure social relationships between baris and how those relationships affect whether a person contracts cholera and how well the cholera vaccine works. In social network analysis, linkages between social actors are modeled. In our study the actors are people with some kinship relationship that will foster movement between physical residences (Wasserman & Faust, 1994). We will create social network metrics using data for all baris based on the Matlab DSS data; the relationships that we will measure are based on family connections. Because all demographic events and internal migrations are noted for all individuals who live in the Matlab area, we can determine how the people in each bari are socially connected to one another. Thus, neighborhood vaccine coverage can be measured not only by spatial proximity and environmental connectivity but also by social connectivity. A combination of these types of interactions that lead to disease transmission is probably best and is the subject of our future research program.
One limitation of this study is that a passive surveillance system was used to enroll the cholera patients, which could produce a variety of selection biases, chief among them, that people reporting tend to be the most severe cases. Since this is an individually randomized trial and we believe the reporting bias would be evenly distributed in both vaccine and placebo groups, any selection bias present should not affect the results of the analysis.
This paper uses new empirical results to test whether or not spatial and environmental information can inform the evaluation of vaccines in Phase III trials. In addition, our work serves as a methodological case study that describes how environmental connectivity analysis methods can be used in vaccine trials. Adding spatial and environmental components to future vaccine trials is feasible. Most vaccine trials begin with an initial population census during which each household is visited by a field worker. Spatial dimensions could be incorporated during this stage by having the field workers collect global positioning system (GPS) locations using inexpensive hand-held receivers when the census questionnaires are being administered. Also, satellite imagery is readily available to represent the aquatic environment. Incorporating these types of geographic methods into vaccine trials will improve vaccine trial evaluation in the future.
Acknowledgments
Funded by National Institutes of Health, Grant # 1R03AI53214-01 and National Science Foundation, Grant # BCS 0323131
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael Emch, University of North Carolina, Chapel Hill, NC UNITED STATES.
Mohammad Ali, International Vaccine Institute.
Elisabeth D Root, University of North Carolina at Chapel Hill.
Mohammad Yunus, International Centre for Diarrhoeal Disease Research, Bangladesh.
LITERATURE CITED
- Ali M, Emch M, Yunus M, Sack D, Lopez AL, Holmgren J, Clemens J. Vaccination of Adult Women against Cholera Protects Infants and Young Children in Rural Bangladesh. The Pediatric Infectious Disease Journal. 2008;27(1):33–37. doi: 10.1097/INF.0b013e318149dffd. [DOI] [PubMed] [Google Scholar]
- Ali M, Emch M, von Seidlein L, Yunus M, Sack DA, Holmgren J, Rao M, Clemens JD. Herd immunity conferred by killed oral cholera vaccines in Bangladesh. Lancet. 2005 Jul 2–8;366(9479):44–9. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- Ali M, Emch M, Ashley C, Streatfield PK. Implementation of a medical geographic information system: Concepts and uses. Journal of Health, Population, and Nutrition. 2001;19(2):100–110. [PubMed] [Google Scholar]
- Bolker BM, Grenfell BT. Impact of vaccination on the spatial correlation and persistence of measles dynamics. Proceedings of the National Academy of Sciences of the USA. 1996;93(22):12648–12653. doi: 10.1073/pnas.93.22.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Rao MR, Chakraborty J, Khan MR, Kay B, Ahmed F, Banik AK, van Loon FP, Yunus M. Evidence that inactivated oral cholera vaccines both prevent and mitigate Vibrio cholerae O1 infections in a cholera-endemic area. Journal of Infectious Diseases. 1992;166(5):1029–34. doi: 10.1093/infdis/166.5.1029. [DOI] [PubMed] [Google Scholar]
- Clemens JD, van Loon F, Sack DA, Chakraborty J, Rao MR, Ahmed F, Harris JR, Khan MR, Yunus M, Huda S. Field trial of oral cholera vaccines in Bangladesh: Serum vibriocidal and antitoxic antibodies as markers of the risk of cholera. Journal of Infectious Diseases. 1991;163(6):1235–42. doi: 10.1093/infdis/163.6.1235. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Harris JR, van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N. Field trial of oral cholera vaccines in Bangladesh: Results from three-year follow-up. Lancet. 1990a;335(8684):270–3. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Chakraborty J, Rao MR, Ahmed F, Harris JR, van Loon F, Khan MR, Yunis M, Huda S. Field trial of oral cholera vaccines in Bangladesh: Evaluation of anti-bacterial and anti-toxic breast-milk immunity in response to ingestion of the vaccines. Vaccine. 1990b;8(5):469–72. doi: 10.1016/0264-410x(90)90248-k. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Huda S, Ahmed F, Gomes J, Rao MR, Svennerholm AM. ABO blood groups and cholera: New observations on specificity of risk and modification of vaccine efficacy. Journal of Infectious Diseases. 1989a;159(4):770–3. doi: 10.1093/infdis/159.4.770. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Harris JR, Kay BA, Chakraborty J, Sack DA, Ansaruzzaman M, Rahman R, Stanton BF, Khan MU, Khan MR. Oral cholera vaccines containing B-subunit-killed whole cells and killed whole cells only. II. Field evaluation of cross-protection against other members of the Vibrionaceae family. Vaccine. 1989b;7(2):117–20. doi: 10.1016/0264-410x(89)90048-0. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Stanton BF, Harris JR, Chakraborty J, Sack DA, Rao MR, Ahmed F, Ansaruzzaman M, Yunus MD, Svennerholm AM. Exclusion of clinically atypical or microbiologically mixed diarrhoeal episodes from outcome events in a field trial of oral cholera vaccines. International Journal of Epidemiology. 1989c;18(2):440–5. doi: 10.1093/ije/18.2.440. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Stanton BF, Ali M, Ahmed F, Yunus M, Kay BA. Impact of B subunit killed whole-cell and killed whole-cell-only oral vaccines against cholera upon treated diarrhoeal illness and mortality in an area endemic for cholera. Lancet. 1988a;1(8599):1375–9. doi: 10.1016/s0140-6736(88)92189-7. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Harris JR, Sack DA, Chakraborty J, Ahmed F, Stanton BF, Khan MU, Kay BA, Huda N, Khan MR. Field trial of oral cholera vaccines in Bangladesh: Results of one year of follow-up. Journal of Infectious Diseases. 1988b;158(1):60–9. doi: 10.1093/infdis/158.1.60. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Neogy PK, Stanton B, Huda N, Khan MU, Kay BA, Khan MR. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: Results of a large-scale field trial. Journal of Infectious Diseases. 1988c;158(2):372–7. doi: 10.1093/infdis/158.2.372. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Harris JR, Sack DA, Chakraborty J, Ahmed F, Stanton BF, Huda N, Khan MR, Khan MU, Kay BA. Field trial of oral cholera vaccines in Bangladesh. Southeast Asian Journal of Tropical Medicine and Public Health. 1988d;19(3):417–22. [PubMed] [Google Scholar]
- Clemens JD, Stanton BF, Chakraborty J, Sack DA, Khan MR, Huda S, Ahmed F, Harris JR, Yunus M, Khan MU. B subunit-whole cell and whole cell-only oral vaccines against cholera: Studies on reactogenicity and immunogenicity. Journal of Infectious Diseases. 1987;155(1):79–85. doi: 10.1093/infdis/155.1.79. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Jertborn M, Sack D, Stanton B, Holmgren J, Khan MR, Huda S. Effect of neutralization of gastric acid on immune responses to an oral B subunit, killed whole-cell cholera vaccine. Infectious Diseases. 1986a;154(1):175–8. doi: 10.1093/infdis/154.1.175. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Stanton BF, Kay BA, Khan MU, Yunus M, Atkinson W. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986b;2(8499):124–7. doi: 10.1016/s0140-6736(86)91944-6. [DOI] [PubMed] [Google Scholar]
- Codeço CT. Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infectious diseases. 2001;1:1. doi: 10.1186/1471-2334-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist EP, Kauth RJ. The tasseled Cap De-Mystified. Photogrammetric Engineering and Remote Sensing. 1986;52(1):81–86. [Google Scholar]
- Durham LK, Longini IM, Jr, Halloran ME, Clemens JD, Nizam A, Rao M. Estimation of vaccine efficacy in the presence of waning: Application to cholera vaccines. American Journal of Epidemiology. 1998;147(10):948–59. doi: 10.1093/oxfordjournals.aje.a009385. [DOI] [PubMed] [Google Scholar]
- Emch M, Ali M, Yunus M, Sack D, Acosta C, Clemens JD. Efficacy calculation in randomized vaccine trials: Global or local measures? Health & Place. 2007;13:238–248. doi: 10.1016/j.healthplace.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Emch M, Ali M, Yunus Park JK, Yunus M, Sack D, Clemens JD. Neighborhood-level ecological correlates of cholera vaccine protection. International Journal of Epidemiology. 2006;35:1044–1050. doi: 10.1093/ije/dyl100. [DOI] [PubMed] [Google Scholar]
- Emch M, Feldacker C, Islam MS, Ali M. Seasonality of cholera from 1974 to 2005: a review of global patterns. International Journal of Health Geographics. 2008;7(31) doi: 10.1186/1476-072X-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emch ME. Diarrheal disease risk in Matlab, Bangladesh. Social Science and Medicine. 1999;49:519–30. doi: 10.1016/s0277-9536(99)00146-x. [DOI] [PubMed] [Google Scholar]
- Hanneman RA. Introduction to social network methods. Riverside, California: Department of Sociology, University of California at Riverside; 2001. [Google Scholar]
- Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque ASG, Colwell R. Climate and infectious disease: Use of remote sensing for detection of Vibrio colerae by indirect measurement. Proc Nat Acad Science. 2000;97(4):1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. Network Epidemiology: A Handbook for Survey Design and Data Collection. Oxford: Oxford University Press; 2004. [Google Scholar]
- Sack DA, Clemens JD, Huda S, Harris JR, Khan MR, Chakraborty J, Yunus M, Gomes J, Siddique O, Ahmed F. Antibody responses after immunization with killed oral cholera vaccines during the 1985 vaccine field trial in Bangladesh. Journal of Infectious Diseases. 1991;164(2):407–11. doi: 10.1093/infdis/164.2.407. [DOI] [PubMed] [Google Scholar]
- van Loon FP, Clemens JD, Chakraborty J, Rao MR, Kay BA, Sack DA, Yunus M, Ali M, Svennerholm AM, Holmgren J. Field trial of inactivated oral cholera vaccines in Bangladesh: Results from 5 years of follow-up. Vaccine. 1996;14(2):162–6. doi: 10.1016/0264-410x(95)00122-h. [DOI] [PubMed] [Google Scholar]
- Wasserman S, Faust K. Social network analysis: Methods and applications. Cambridge: Cambridge University Press; 1994. [Google Scholar]