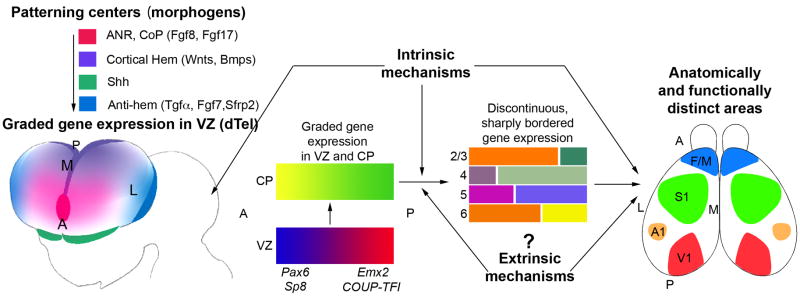
Figure 1. Patterning centers and graded transcription factors drive arealization of the neocortex.
The initial, tangential gradients of transcription factors (TFs) in the ventricular zone (VZ) are established by signaling molecules/morphogens secreted from telencephalic patterning centers, such as Fgf8 and Fgf17 from anterior neural ridge (ANR), which later becomes the commissural plate (CoP), and Wnts and BMPs from the cortical hem. The antihem is a putative patterning center identified based on its expression of secreted signaling molecules (e.g. Tgfα, Fgf7, Sfrp2, as well as Neurogulin 1 and 3) with known patterning functions. A fourth telencephalic patterning center is defined by the expression domains of sonic hedgehog (Shh) in ventral telencephalon, but it does not have defined roles in dorsal telencephalic (dTel) patterning. The graded expression of certain TFs, such as Pax6, Emx2, COUP-TFI and Sp8, imparts positional or area identities to cortical progenitors which is imparted to their neuronal progeny that form the cortical plate (CP). The CP also initially exhibits gradients of gene expression that are gradually converted to distinct patterns with sharp borders. Coincident with this process, distinct cortical layers (2–6), and the anatomically and functionally distinct areas seen in the adult (M1, S1, A1, V1), differentiate from the CP. Genes that are differentially expressed across the cortex are often expressed in different patterns in different layers, suggesting that area-specific regulation of such genes is modulated by layer-specific properties, and questions the definition of area identity. Although the initial establishment of the graded gene expression in the embryonic CP is controlled by mechanisms intrinsic to the telencephalon, the more complex differentiation patterns established postnatally might be controlled in part by extrinsic mechanisms, for example, TCA input and the sensory activity that it relays from the periphery to the cortex. The figure is modified from [64].