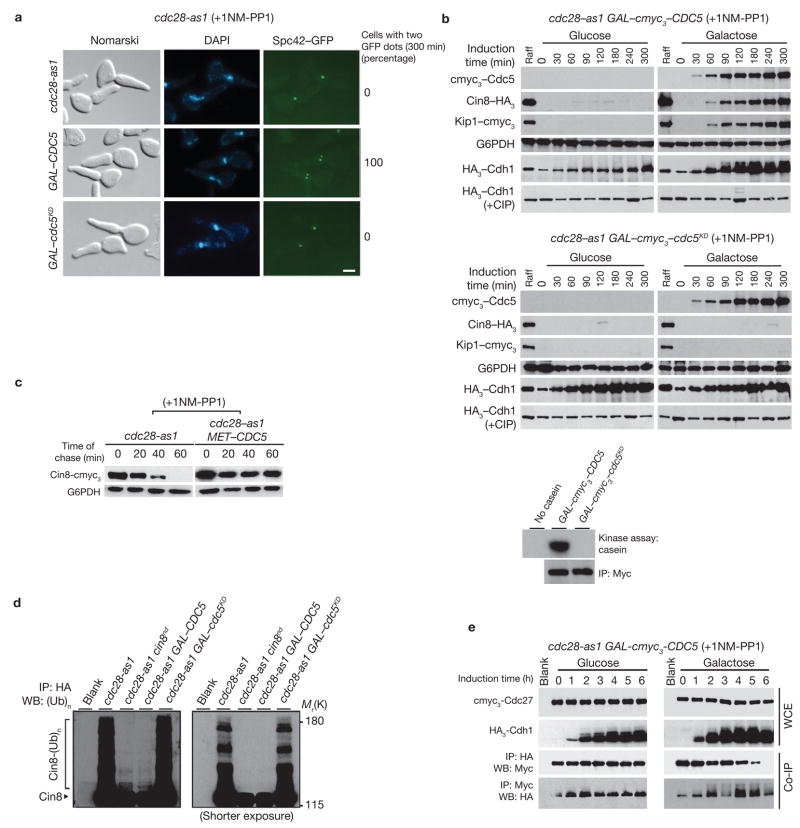
Figure 1.
Ectopic expression of Cdc5 causes hyperphosphorylation of Cdh1 and SPB separation. (a) cdc28-as1 cells carrying GAL–CDC5 (US5255) or GAL–cdc5KD (kinase-dead; US5360) were synchronized by α-factor treatment in YEP+Raff and released into YEP+Raff+Gal containing 1NM-PP1 at 24 °C for 300 min. Numbers indicate percentage of cells with two Spc42–GFP dots at 300 min. The scale bar represents 2 μm. (b) Top panel: G1-synchronized cdc28-as1 cells carrying GAL–cmyc3–CDC5 (US5233) were released into YEP+Raff+Gal containing 1NM-PP1 for 6 h and cells were collected at the indicated times. Western blots show Cin8–HA3 and Kip1–cmyc3 levels, and immunoprecipitated HA3–Cdh1 (with and without alkaline phosphatase (CIP)). cdc28-as1 cells carrying GAL–cmyc3–cdc5KD (US5371) were treated as described for the top panel. Bottom panel: cmyc3–Cdc5 kinase was immunoprecipitated from cdc28-as1 cells carrying either GAL–cmyc3–CDC5 or GAL–myc3–cdc5KD and was used in an in vitro assay to determine kinase activity using casein as a substrate. (c) cdc28-as1 MET–CDC5 cells carrying GAL–CIN8–cmyc3 (US5310) were grown in Raff+Met medium containing α-factor for 2 h at 24 °C and then transferred to Raff+Gal+Met medium containing α-factor for 90 min to induce Cin8 expression. Cells were then released at 24 °C into methionine-deficient glucose medium containing 1NM-PP1 and cycloheximide (1 mg ml−1) to inhibit protein synthesis). The fate of Cin8 pulse was determined by western blotting. (d) G1-synchronized cdc28-as1, cdc28-as1 expressing non-degradable version of Cin8 (cin8nd) from endogenous promoter, cdc28-as1 GAL–CDC5 and cdc28-as1 GAL–cdc5KD (US5312, US5314, US5315, US5341) were released into medium containing 1NM-PP1 and the proteasome inhibitor MG132. All except cin8nd-carrying strains harboured endogenously-tagged CIN8–HA3. Ubiquitin conjugates of immunoprecipitated Cin8 were separated on a 6% SDS gel and detected by anti-ubiquitin antibodies. The blot on the right shows a shorter exposure. (e) G1-synchronized cdc28-as1 GAL–CDC5 cells carrying endogenously-tagged cmyc3–CDC27 and HA3–CDH1 (US5355) in raffinose medium were released into either glucose or galactose medium containing 1NM-PP1. Immunoprecipitates were analysed by western blotting. Full-length blots for b, c and e are presented in Supplementary Information, Fig. S5–1.