Figure 1. Structure of AMPK.
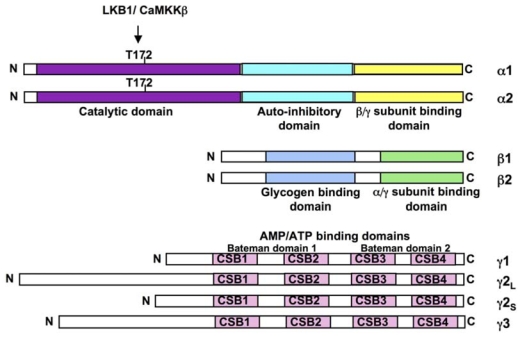
The mammalian AMPKα (α1 and α2), AMPKβ (β1 and β2) and AMPKγ (γ1, γ2 short form, γ2 long form and γ3) subunits are shown. The α-subunits contains the Thr172 residue that must be phosphorylated (P) by upstram kinases for activity and an autoinhibitory sequence domain that inhibits the activity of the kinase domain. The C-terminal domain is required for binding the β- and γ-subunits. The β-subunits contains central glycogen-binding domains and C-terminal domain that is required for binding the α- and γ subunits. The three γ-subunit isoforms have variable N-terminal domains and four conserved cystathionine beta-synthase motifs (CBS1–4). The CBS motifs act in pairs to form two Bateman domains that bind AMP or ATP.
