Abstract
Protein kinase CK2 is a serine/threonine kinase known to phosphorylate numerous substrates. CK2 is implicated in several physiologic and pathologic processes, particularly in cancer biology. CK2 is comprised of several subunits, including CK2α, CK2α′ and CK2β. Inactivation of CK2α′ leads to chromatin degeneration of germ cells, resulting in male sterility. To identify additional targets of CK2α′ in testes and to determine the role of CK2α′ in germ cell nuclear integrity, GST pull-down and protein–protein interaction assays were conducted. A novel testis-specific gene, CKT2 (CK2 Target protein 2), was found whose product interacts with and is phosphorylated by CK2 in vitro and in vivo. CKT2 is a 30.2 kDa protein with one coiled-coil domain and six putative phosphorylation sites. High expression of CKT2 correlated with chromatin condensation of spermatids in murine testes. Findings reported herein demonstrate that CKT2 is a target protein of native CK2α′ in testes and suggest that CKT2 plays a role in chromatin regulation of male germ cells.
INTRODUCTION
Protein Kinase CK2 is a serine/threonine phosphotransferase that exists primarily as a tetrameric structure with two catalytic subunits, CK2 alpha (CK2α) and CK2 alpha prime (CK2α′), and two regulatory CK2 beta (CK2β) subunits (1). CK2α and CK2α′ are gene products of Csnk2a1 and Csnk2a2, respectively. A third isoform of the catalytic subunit, called CK2α′′, is a larger variant of CK2α that has a translated Alu sequence at the C-terminus (2). The human genome contains four CK2 loci, corresponding to three active genes, coding for the catalytic α and α′ subunits, one regulatory β subunit, and a processed α subunit pseudogene (3). CK2 has the unique ability to utilize GTP as a phosphate donor in place of ATP (4).
CK2 has been reported to phosphorylate many physiological substrates (4). More than one-third of the known 300+ protein substrates phosphorylated by CK2 are involved in gene expression and protein synthesis, either as transcription factors, effectors of DNA/RNA structure, or translation elements. Substrates for CK2 also function as signalling proteins and those essential for viral replication (5,6). Candidate cellular CK2 substrates include proto-oncogene products such as c-Myc, c-Myb and c-Jun; tumour-suppressor gene products such as p53 and BRCA1; transcriptional regulators such as Max, Cut, PU.1/IRF4 and Six1; and components of the canonical Wnt pathway (7).
CK2 has been implicated in diverse cellular processes such as cell cycle regulation and cell growth, circadian rhythms, apoptosis, regulation of cell polarity, embryonic development, cell morphology and the cytoskeleton (4,8,9). Increased constitutive activity of CK2 has also been implicated in the pathogenesis of various infectious diseases, neurodegenerative diseases and cardiovascular disorders (10).
The best known pathogenic role for CK2 has been in neoplastic growth and high CK2 activity has been observed in numerous cancers (11). Consistent with its ability to enhance cancer growth, CK2 promotes aberrant activation of nuclear factor-kappaB, suppression of cellular apoptosis and survival of breast cancer cells (12,13). It also induces lymphocyte transformation in transgenic mice, collaborates with Ha-Ras in fibroblast transformation, and is involved in the pathobiology of androgen-dependent and -independent prostate cancer (14,15). CK2 can exert an anti-apoptotic role by protecting regulatory proteins from caspase-mediated degradation and counteracting caspase cleavage (6). As a result of its cancer-promoting properties, CK2 has been considered to be a target of anti-neoplastic treatments (16,17).
The catalytic subunits of CK2—CK2α and CK2α′—are paralog proteins. Typically, the evolution of paralog proteins is associated with a functional specialization (18). CK2α and CK2α′ exhibit approximately 90% identity in their catalytic domains, but mainly differ in their C-terminal region (18). CK2α’ and CK2α are significantly different with respect to some well-known CK2 properties such as autophosphorylation and supra-molecular aggregation (18). Abundance of CK2α′-containing holoenzyme in certain tissues suggests an important role of CK2α′ in these tissues (18,19). Indeed, CK2α is the more abundant CK2 subunit in developing embryos ad in contrast to CK2α′−/− embryos, CK2α−/− embryos die in mid-gestation (20). A pro-neoplastic role for CK2α′ has been demonstrated in that human osteosarcoma U2-OS cell proliferation is inhibited following the over-expression of a kinase inactive variant of CK2α′, but not of CK2α (15).
CK2α′ is found predominantly in testis (21,22). Our previous studies showed that male mice lacking CK2α′ were infertile and exhibited an abnormal shape in their spermatid nuclei (23). In addition, a significant number of germ cells in the CK2α′−/− mice were eliminated by apoptosis (23). Moreover, male germ cells at various steps of differentiation showed unique perturbations of chromatin and nuclear envelope in the CK2α′−/− mice (24). Therefore, the phenotype of the CK2α′−/− mice suggests that CK2α′ has a role in cell survival, in maintaining nuclear integrity of male germ cells, and raises the question of whether dysregulated expression of CK2α′ may be involved in testicular malignancy.
Compared to other CK2 subunits, significantly less is known about CK2α′. To better understand the testis-specific function of CK2α′, the yeast two-hybrid technique was used to identify protein(s) that can interact with testicular CK2α′. As we will show, a new gene designated CKT2 (CK2 Target protein 2) was identified in the mouse chromosome 16 and specifically expressed in mouse testes. Furthermore, we will show that CKT2 is a target protein of CK2α′, is phosphorylated by CK2, and is present in the nuclei of mouse spermatids during chromatin condensation.
MATERIALS AND METHODS
Detailed descriptions of the following Materials and Methods are given in the Supplementary data: reagents and molecular tools, and Southern blot.
Construction of plasmids for two-hybrid experiments
The ORF region of CK2α′, CK2α and CK2β were cloned into the Gal4 DNA-binding domain vector pGBKT7. ORF N- and C-terminal regions of CKT2 were cloned in Gal4 transcriptional activation domain vector pACT2; all plasmids were constructed by standard molecular biology methods (25) and confirmed by sequencing.
Yeast two-hybrid screen and cDNA isolation
The full length murine CK2α′ cDNA was cloned into the pGBKT7 vector in the in-frame fusion of CK2α′ with the DNA-binding domain of the yeast GAL4 protein. A mouse testis cDNA library containing 4.5 × 106 independent clones (Clontech Laboratories Inc.) was cloned into pACT2 to produce fusions between the targeted proteins and the cDNA activation domain of GLA4. Screening was performed by sequential transformation of bait and library vectors in Saccharomyces cerevisiae strain Y190 used for the screening assay containing his, ade and lacZ reporter genes under the control of a GAL4-responsive upstream activation site. Transformants were plated on SC-Leu-Trp-Ade-His- medium and incubated at 30°C for up to 3–5 days. A cDNA for the CKT2 gene was obtained from 2 × 106 transformants of mouse testis cDNA in pACT2 and using CK2α′ as bait in pGBKT7 clones. A testis cDNA library in λgt11 was screened for the selected clone 8. For the localization of CKT2 gene, the mouse BAC genome DNA was used (Clontech Laboratories Inc.). The DNA sequencing was performed at Yale University, and the GenBank database was searched by using the BLAST program (National Center for Biotechnology Information, Bethesda, MD, USA).
Isolation of RNA and RT–PCR
Total RNA was isolated from different organs of adult mice as described previously (26). RT–PCR using the Advantage RT–PCR Kit (Promega Corp.) was performed according to the manufacturer's instruction. The following primers were used: primer of sense mouse β-actin 5′-GCTGTGTTCCCATCCAT-CGTGG-3′ (nt1, 875–1896) and antisense mouse β-actin 5′-GACGCATGATGGCG-GTGTGGCA-3′ (nt 2561–2540), CKT2 sense primer 5′-CAATTCCATCTCCAAGTCTAC-3′ (nt 451–472) and CKT2 antisense primer 5′-TCAGGATTTCTCATTTTGAAAC-3′ (nt 1339–1317).
Recombinant proteins
To produce the CKT2 and CK2 subunit recombinant protein, the coding region of CKT2 and CK2 subunit cDNA was amplified by PCR using primers representing the first, middle and last 20 nucleotides of CKT2 and CK2. The upstream and downstream primers contained at their 5′-ends a BamHI and EcoRI restriction enzyme recognition site, respectively. The PCR product was unidirectionally cloned into pGEX2 vector to create pGCKT2 (1–276), pGCKT2-n (1–137) and pGCKT2-c (138–276) GST fusion vectors. For expression, Escherichia coli BL21 were used. The cells were first grown at 30°C to an optical density of 0.8, induced with 0.5 mM final concentration of isopropyl-β-d-thiogalactopyranoside (IPTG), and incubated at 30°C for another 5 h before harvesting the culture. The recombinant protein was purified on glutathione-SepharoseTM 4B (Amersham Biosciences AB, Uppsala Sweden), and the protein concentration was determined by the Bradford Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA).
Antibody preparation
The (1–276) and (138–276) CKT2 cDNA were constructed in pGEX2 vector (Pharmacia). The recombinant proteins were expressed in Escherichia coli BL21 and purified by glutathione-sepharose beads (Pharmacia). The recombinant proteins were injected into female mice to produce monoclonal antisera against CKT2. For polyclonal antibodies, peptides encompassing 83–102 and 257–276 CKT2 amino acids were synthesized; conjugated to KLH and injected into rabbits. Antisera were purified by affinity to CKT2 peptide.
In situ hybridization
Paraffin-embedded testicular sections of C57BL/6J adult male mice were fixed in freshly prepared 4% paraformaldehyde in PBS. The cDNA fragment of CKT2 was cloned into vector pBluescript II KS+ containing two RNA transcription promoters, T3 and T7, to be named pBCKT2. The sense probe was synthesized using T3 RNA polymerase and the plasmid was linearized with XhoI, whereas the antisense probe was synthesized using T7 RNA polymerase and the plasmid was linearized with EcoRI. In situ hybridization (ISH) was performed using 35S-labeled antisense or sense probes transcribed from full-length cDNA for CKT2 by using a Superscript kit (Promega Corp.). Tissue section ISH was performed as previously described (26).
Immunohistochemistry
Testes were decapsulated, fixed for 3 h in 4% paraformaldehyde in PBS (phosphate-buffered saline) and then incubated in sucrose solutions of increasing concentration (12%, 15% and 18%) before freezing and sectioning. Sections were incubated with anti-CKT2 antibody (diluted 1/100). Controls were performed as for immunoelectron miscroscopy (see below). Immunohistochemical labeling was performed with the three-step immunoperoxidase technique using the biotin-avidin system (Vector Laboratories, Burlingame, CA, USA). Amino-ethyl-carbazole was used as the chromogen. Sections were counterstained with Harris hematoxylin and mounted in aqueous medium (Glycergel, Dako Corp., Carpinteria, CA, USA).
Immunoelectron microscopy
Testes from 2- to 3-month-old C57BL/6J mice were fixed with 4% paraformaldehyde at 4°C overnight for post-embedding immunolabeling. The samples were washed several times in 1×PBS and subsequently dehydrated with a series of graded ethanol solutions, then embedded in Lowicryl K4M and crosslinked under UV for 3 days at 4°C. Ultrathin (900 nm) sections were dehydrated with PBT/nonfat milk [PBS, pH 7.2, 0.05% (v/v) Tween 20, 0.1% (w/v) nonfat milk] for 15 min and incubated with anti-CKT2 antibodies for 2 hrs, extensively washed with PBT, and incubated for 1 h with secondary IgG antibody labeled with colloidal gold (15 nm gold particles; BBInternational, Cardiff, UK), both diluted 1/100 in PBS/nonfat milk. Grids were then washed in PBT and rinsed in distilled water. Control experiments were performed by (i) omission of the anti-CKT2 antibodies, (ii) replacement of anti-CKT2 antibodies by preimmune serum and (iii) preincubation of CKT2 antibodies with the specific CKT2 peptide. Counterstaining was omitted. The ultrastructural examination of the tissues was performed using a JEOL (JEM 100CX II) (Jeol Ldt, Tokyo, Japan) electron microscope operating at 80 Kv, and photographs taken on Kodak electron microscopy film.
β-galactosidase filter and liquid assay
Single colonies were picked and transferred to a Whatman filter paper (No. 5) for β-galactosidase assay; the filter and liquid assay were performed as previously described (26).
Cell culture and transfection
HEK293 cells were cultured in Fugene's medium (Boheringer Mannheim, Germany) following the company's description and supplemented with 10% fetal bovine serum (Invitrogen, BV NV Leek, Netherlands) at 37°C in a 5% CO2 incubator. A Flag tag was fused to CKT2 and subcloned into pcDNA3 expression vector (Invitrogen) and the DNA construct was verified by direct sequencing. The cells were transfected using the calcium phosphate precipitation method as described previously (27), using 50 μg of DNA per 8 × 106 cells in a 15-cm diameter plate. Co-transfection experiments used equivalent amounts of DNA for each plasmid unless otherwise indicated. Cells were washed thoroughly with PBS and replenished with fresh medium at 16–18 h after transfection. After 3 days, the cells were fixed in 2% paraformaldehyde and stained with M2 anti-Flag antibody (Sigma Aldrich). Following addition of a secondary anti-mouse IgG antibody coupled to FITC, immunofluorescence was detected.
Glutathione S-transferase pull-down assay
GST pull-down assay was performed with purified GST-CKT2 fusion protein. Method 1 (GST-CKT2 plus labeled 35S-CK2α′ or 35S-CK2α): CK2α′ or CK2α subunits labeled with [35S] methionine were synthesized by coupled transcription translation with wheat germ extract from a TnT kit (Promega Corp.) and with T7 polymerase according to manufacturer's instructions. For in vitro binding, 20 μl of the reaction was added to 200 μl of binding buffer (100 mM KCl, 10% glycerol, 5 mM EDTA/20 mM Hepes, pH 7.6, 0.02% NP40, 1 mM DTT, 5 mg/ml BSA) followed by 10 μl of glutathione-agarose beads with bound GST or GST-CKT2 and the mixture was incubated at 4°C. The beads were washed three times with binding buffer. Bound proteins were eluted with 2× SDS–PAGE sample buffer and were resolved by 12% SDS–PAGE. 35S-labeled bands were detected by X-ray autoradiography using a PhosphoImager 425 (Molecular Dynamics, Sunnyvale, USA). Method 2 (GST-CKT2 plus testicular lysate): 20 μl of glutathione-agarose beads bound to GST-CKT2 or GST were incubated at 4°C with 30 μl of testicular lysates from either the wildtype or CK2α′ null mice. The beads were washed three times with binding buffer. The bound proteins were eluted with 2× SDS–PAGE sample buffer, resolved by 12% SDS–PAGE, and the separated proteins transfered to PVDF membranes (Boehringer, Mannheim, Germany) in buffer containing 20 mM Tris–HCl, 150 mM glycine, pH 8.9. The membranes were blocked for 1 h in buffer [0.05% (v/v) Tween 20 in PBS pH 7.4] with 5% (w/v) dry milk, washed in PBS and incubated for 1 h with CK2α and CK2α′ antibodies (1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following washing in binding buffer, the membranes were decorated with HRP-conjugated secondary antibody, washed and visualized by ECL according to the manufacturer's instructions (Amersham Buschler Gmbh & Co. KG, Braunschweig, Germany).
In vitro kinase assays
The specific CK2 inhibitor peptides were ETERRREEETEEE and DSDRRRDDDSDDD (Gift from Dr D.C. Seldin, Boston, MA, USA). CK2 peptide without a phosphorylation site (RETSALLRYFQTKFQNEKS) was used as control. The GST-CKT2 fusion proteins were expressed in recombinant Escherichia coli and immobilized on glutathione-agarose beads. GST-CKT2 beads were then incubated with 10 μg of protein lysates of mouse testes (wildtype or CK2α′ mutant) at 4°C for 2 h, then washed three times with buffer (20 mM Tris–HCl at pH 7.5, 200 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1% Triton X-100, 0.1 mM dithiothreitol, 1 mM PMSF protease inhibitor). The samples were then incubated in 400 μl buffer (100 mM Tris, pH 8.0 20 mM MgCl2, 100 mM NaCl, 50 mM KCl and 100 μM ATP containing 5 μCi of γ-32P-ATP) at 30°C for 30 min. The kinase reactions were terminated by washing the samples twice and re-suspending the samples in SDS sample buffer. Then the samples were boiled for 5 min and the proteins were resolved by SDS–PAGE. Phosphorylated proteins were visualized and quantified by PhosphoImager analysis.
Immunoprecipitation, western blot analysis and CK2 kinase assay
Immunoprecipitation
Testicular cell extracts of C57BL/6J mice were prepared by griding mice testicles with a lysis buffer containing phosphatase and protease inhibitors [50 mM Tris–HCl, pH 7.5, containing 0.5% (v/v) Nonidet P-40, 1 mM EDTA, 150 mM NaCl; 5 µg/ml aprotinin, 5 µg/ml leupeptin, 2 mM Na3VO4, 1 mM PMSF]. After centrifuging the lysates at 20 800g at 4°C for 15–30 min, the protein concentrations were determined. One hundred µg aliquots of the testicular lysates were incubated with the anti-CKT2 antibody (2 µg/ml) or the anti-CK2α′ antibody (2 µg/ml) overnight at 4°C with gentle rotary mixing. The protein-antibody complexes were isolated by the addition of 20 µl protein A-sepharose beads (Santa Cruz), followed by gentle rotary mixing for 2 h at 4°C. The beads were then washed three times with wash buffer [20 mM Tris–HCl at pH 7.5, 200 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1% (v/v) Triton X-100, 1 mM DTT, 1 mM PMSF].
Western blot
The bead pellets obtained were resuspended in 30 µl 1× Laemlli/DTT buffer, incubated for 5 min at 95°C, and then separated by SDS–PAGE. The separated proteins were transferred onto PVDF membranes (Millipore) by electroblotting. The blotted membranes were blocked with 1×TBS containing 5% (w/v) nonfat milk and 0.05% (w/v) Tween-20 for 1 h at room temperature to reduce any nonspecific interactions. After multiple washings with 1×TBS, the membrane was incubated with anti-CK2α′ antibody for 1 hr in 1×TBS containing 5% (w/v) nonfat milk. After additional washes, the membranes were incubated with HRP-conjugated secondary antibodies (1:2000) for 60 min at room temperature in 1×TBS containing 5% (w/v) nonfat milk. After additional washings, the membranes were developed using an enhanced chemiluminescence detection kit (Cell Signaling Technology) and XAR sensitive film (Amersham Biosciences).
CK2 kinase assay
The CK2α′ immunoprecipitated pellets obtained were resuspended in 25 µl of kinase buffer (100 µM sodium orthovanadate, 100 mM Tris–HCl (pH 8), 100 mM sodium chloride, 20 mM MgCl2, 50 mM KCl, 100 µM ATP containing 5 µCi of [γ-32P] ATP and 5 mg/ml CK2 casein). Kinase reactions were incubated for 15 min at 37°C and stopped by the addition of 10 µl of reducing solubilizing buffer [50 mM Tris–HCl, (pH 6.8), 100 mM DTT, 2% (w/v) SDS, 0.1% (w/v) bromphenol blue, 10% (v/v) glycerol]. Samples were boiled for 10 min and subjected to SDS–PAGE. After electrophoresis, the gels were fixed for 20 min in a solution containing 40% (v/v) methanol and 10% (v/v) acetic acid. These were washed once with distilled water before being dried under vacuum and subjected to autoradiography.
Metabolic labeling
HEK293 cells (500 000 cells/well in a 6-well tissue culture plate) were transfected with pcDNA3 or pcDNA3-CKT2 plasmid using Lipofectamine 2000 according to manufacturer's instructions (Invitrogen) and then cultured for 24 h. The cells were then washed twice with phosphate-free DMEM supplemented with 10% heat-inactivated FBS. The cells were then incubated with 1 mCi/ml [32P]-orthophosphate in the same medium for 6 h (28). The cells were lysed, centrifuged, and the supernatant of the cell lysates were immunoprecipitated with 1 µg anti-CKT2 antibody per condition. The immunoprecipitated CKT2 was separated by SDS–PAGE, transferred to a nitrocellulose membrane and subjected to autoradiography. Total CKT2 was qualitatively determined by immunoblotting the same membrane with 1:1000 anti-CKT2 antibody.
RESULTS
Isolation of murine CK2α′ interacting partners
To identify proteins that can interact with CK2α′, a yeast two-hybrid screening was performed using the GAL4 recognition site to regulate expression of his, Ade and LacZ reporter genes. The GAL4-DB-CK2α′ fusion protein alone did not activate transcription of the reporter genes (GAL4-DB: GAL4 DNA-binding domain). The GAL4-DB-CK2α′ plasmid pGBKT7 was co-transformed with the GAL4-AD-cDNA library of mouse testis pACT2 into the AH109 strain of S. cerevisiae (GAL4-AD: GAL4 activation domain). Transformants were plated on SC-Leu-Trp-Ade-His-selective medium. Fifteen candidates positive for CK2α′ were obtained from 2 × 106 yeast cells co-transformed with murine testicular cDNA library in pACT2 and with CK2α′ in pGBKT7 plasmid as bait. Sequence analysis showed that 13 of them were CK2β, one was CKT1 (unpublished data), and one was a new gene designated CKT2, which was further analyzed.
Characteristics of CKT2
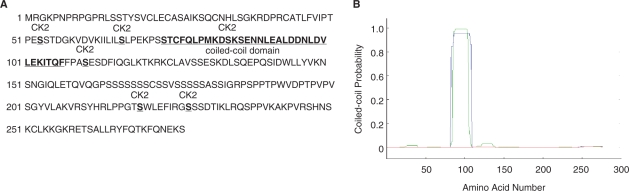
Since a putative translational initiation codon was missing in this cDNA CKT2 clone, a mouse testis cDNA library in λgt11 was screened to isolate corresponding clones and used as a probe. The sequence of the full-length cDNA was accepted in Genbank (accession number NM_173861; NCBI interim symbol is Ckt2). Analyses of CKT2 DNA sequence showed that the ORF of CKT2 encodes a 276 amino acid protein corresponding to a 30.2 kDa gene product (Figure 1A). The first start codon (ATG) of the ORF began at position 511 and was preceded by an in-frame stop codon at position 21 to 24 and by a purine (A) at position 3. The deduced amino acid sequence of CKT2 showed a 23.9% homology with c-Myc (Supplementary Figure 1). According to the consensus sequence (S/T)XX(D/E) that is phosphorylated by CK2, analysis of the CKT2 sequence revealed six putative CK2 phosphorylation sites (Figure 1A). A stretch of residues located at position 75-108 exhibited a strong probability of coiled-coil formation, as confirmed by the use of the coiled-coil domain prediction program (www.ch.embnet.org) (Figure 1B).
Figure 1.
(A) CKT2 amino acid sequence corresponding to the CKT2 open reading frame. The sequence contains a coiled-coil domain and six putative CK2 phosphorylation sites. The numbers on the left refer to amino acid positions (Genbank Accession No. NM_173861). (B) Prediction of a coiled-coil domain in the CKT2 gene. The x-axis corresponds to the amino acid numbers. The probability of the polypeptide to assume a coiled-coil conformation is plotted on the y-axis.
To localize the CKT2 gene, a Southern blot was performed with BAC mouse genome DNA and cDNA of CKT2 as probe (Figure 2A). A 1.6 kb EcoRI fragment was cloned and sequenced and the CKT2 gene was localized in BAC number 8 of the mouse genome. The sequence of 1.6 kbp EcoRI fragment was compared to CKT2 cDNA nucleotide (Supplementary Figure 2). Mouse Genome Blast database (WU-BLAST 2.0) and Celera Genomics Database indicated that CKT2 was an unknown gene localized to mouse chromosome 16.
Figure 2.
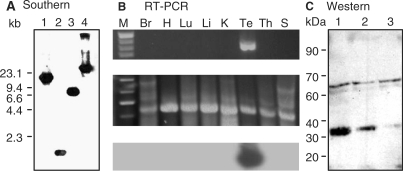
Expression of CKT2 gene and CKT2 protein. (A) Southern blot analysis. Mouse BAC genomic DNA (10 µg) was digested with BamHI (lane 1), EcoRI (lane 2), HindIII (lane 3) and XbaI (lane 4). The fragments were hybridized with a 1.1 kb radiolabeled cDNA probe of CKT2 (nucleotides 242–1342). A 1.6 kb fragment was obtained with EcoRI (arrowhead). The sizes of DNAλ/HindIII markers are indicated on the left. (B) Upper panel: RT-PCR of CKT2 transcripts from total RNA from various mouse tissues. An 880 bp PCR product is present only in mouse testis; Middle panel: RT–PCR of β-actin as control; bottom panel: Southern blot of various mouse tissues with 32P-labeled pCKT2 cDNA fragment excised with EcoR1/Xhol confirms that the RT–PCR product corresponded to the CKT2 gene. Markers (M), brain (Br), heart (H), lung (Lu), liver (Li), kidney (K), testis (Te), thymus (Th) and spleen (S). (C) Western blot of mouse testicular lysates with mouse monoclonal anti-CKT2 antibody. A protein of about 31 kDa is revealed (arrowhead). Amount of protein loaded: lane 1 (30 µg), lane 2 (10 µg), and lane 3 (5 µg). Data shown are representative of three independent experiments.
Expression of CKT2 in mouse testis
Reverse transcription coupled with PCR amplification (RT–PCR) was performed to determine which mouse tissue(s) expressed CKT2. RT–PCR was performed on total mRNA isolated from different tissues to amplify the region encompassing an 880 bp fragment of CKT2 from mouse testis-derived cDNA. As shown in Figure 2B (top panel), an 880 bp RT-PCR product corresponding to CKT2 mRNA was found exclusively in mouse testes. Figure 2B (middle panel) shows β-actin control for the RT–PCR reactions. In order to confirm that this RT–PCR fragment corresponded to CKT2, a Southern blot was performed using a 32P-labeled pCKT2 cDNA fragment excised by EcoRI/XhoI. As shown in Figure 2B (bottom panel), a strong positive signal for CKT2 gene was obtained in the testes. These results show that in the mouse, CKT2 is specifically expressed in the testes.
A western blot of mouse testicular lysates using a mouse monoclonal antibody against CKT2 revealed a protein of about 30–31 kDa, consistent with the size of CKT2 protein as predicted by the ORF of the CKT2 gene (Figure 2C). These findings indicate that the cloned CKT2 cDNA encodes the full-length CKT2 protein.
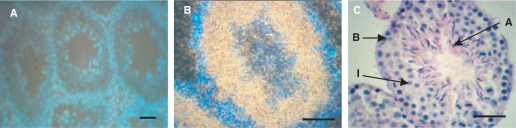
To further investigate CKT2 expression in the mouse testes, we performed in situ hybridization on sections of adult mouse testes, using a 35S-labeled pBCKT2/EcoRI antisense riboprobe or a negative control 35S-labeled pBCKT2/XhoI sense Riboprobe. No signal was present in testis section hybridized with the sense probe (Figure 3A). Sections of the testes hybridized with antisense probe showed a CKT2 mRNA signal localized in the seminiferous tubules, while the signal was absent in the inter-tubular compartment. Higher magnification showed a strong signal in the intermediate compartment of the seminiferous tubules (Figure 3B). A weak signal was located in the basal compartment containing spermatogonia and in the adluminal compartment within condensed spermatids; Figure 3C shows these three compartments in an H & E stained mouse seminiferous tubule. These results indicate that CKT2 was specifically expressed in male germ cells undergoing differentiation at stages of spermatocytes and young spermatids. However, expression of CKT2 mRNA cannot be excluded in Sertoli cells. CKT2 expression in spermatogonia was found to be low, as compared to germ cells at more advanced stages of spermatogenesis and as shown by immunohistochemistry (see below).
Figure 3.
In situ hybridization analysis revealed that CKT2 mRNA was expressed by germ cells in mouse testis. Testicular section hybridized with 35S-CKT2 sense probe (A) or antisense probe (B). Differences in the blue color background between the two figures are due to different magnifications (low in A and high in B) and the color of the silver grain signal was due to adjustment of various filters of the microscope. Hybridization signals of CKT2 were observed in the intermediate compartment of seminiferous tubules. (C) A serial section stained by H&E shows the mouse seminiferous tubule compartments (B: Basal; I: Intermediate; A: Adluminal). Scale bar: 100 µm. Data shown are representative of three independent experiments.
CKT2 localizes to the nuclei of spermatids
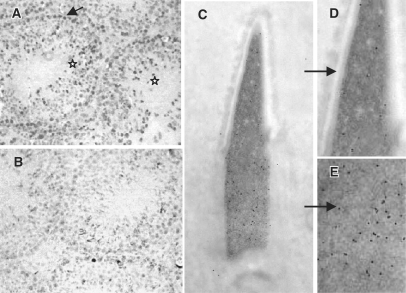
As shown in Figure 4A, mouse testicular sections immunostained with anti-CKT2 antibody revealed that CKT2 was predominantly expressed in the nuclei of spermatids (asterisks) and, to a lesser extent, in the nuclei of some spermatogonia (arrow). Testes incubated with nonimmune IgG revealed absence of staining (Figure 4B). Sub-cellular localization of CKT2 in male germ cells was further investigated by immunogold labeling with anti-CKT2 antibody of mouse testis sections embedded in Lowicryl followed by electron microscopy. As shown in Supplementary Figure 3, CKT2 expression varied with the stage of chromatin condensation. During the initial steps of chromatin condensation, CKT2 labeling was weak in the spermatids (Supplementary Figure 3A) but increased during the intermediate stages of chromatin condensation (Supplementary Figure 3B). In more mature spermatids, the labeling decreased in the apical nuclear region with greater condensed chromatin, as compared to the less condensed posterior region (Figure 4C–E and Supplementary Figure 3C and D). CKT2 was absent in spermatids with fully condensed chromatin (data not shown). These findings indicate a correlation between a high expression of CKT2 and the step of chromatin condensation in spermatids.
Figure 4.
Expression of CKT2 in testicular cells. (A) Immunohistochemistry of testis section with anti-CKT2 antibody; nuclei of spermatogonia (arrow) and spermatids (asterisks). (B) Testis incubated with nonimmune IgG antibody. (C) Immuno-gold labeling for CKT2 of a spermatid at step 13 of differentiation showing that CKT2 is present in the nuclei of spermatids undergoing chromatin condensation. As shown in the magnified views, the labelling is weaker in the more condensed apical nuclear region (D), as compared to the stronger labelling in the less condensed posterior nuclear region (E) (see the labelling pattern of spermatids at different steps of differentiation in Supplementary Figure 3). Data shown are representative of two independent experiments.
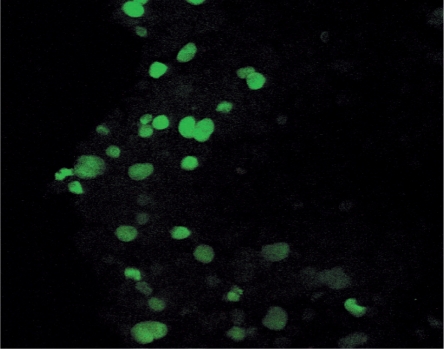
The nuclear localization of CKT2 was confirmed in HEK293 cells transfected with Flag-CKT2. As shown in Figure 5, staining of the transfected HEK293 cells with FITC-labeled anti-Flag antibody revealed that CKT2 was found in the nuclei, confirming that CKT2 is a nuclear protein.
Figure 5.
CKT2 expression in the nuclei of HEK293 transfected cells. The epitope Flag was fused to CKT2 gene and subcloned into pcDNA3 expression vector and transfected into mammalian HEK293 cells, as described in Materials and Methods section. Immunofluorescent staining of transfected cells with FITC-labeled anti-Flag antibody revealed that CKT2 was found predominantly in the nuclei.
CKT2 interacts with the CK2α′ subunit of CK2
In order to determine whether CKT2 interacts with CK2 subunit(s), we used three experimental approaches: yeast two-hybrid assay, glutathione S-transferase (GST) pull-down assay, and co-immunoprecipitation. In the first approach, cDNAs encoding CK2α, CK2α′ or CK2β subunit were each ligated into pGBKT7 vectors using the yeast two-hybrid technique. cDNAs encoding either the full length, the N-terminus, or the C-terminus of CKT2 were ligated into pACT2 vectors in order to express each as a fusion protein with the DNA-binding domain (pGBKT7), or with the transcriptional activation domain (pACT2) of the yeast Gal4 transcription factor. The S. cerevisiae strain HA109, which expresses β-galactosidase activity under the control of a Gal1 promoter upon binding by the Gal4 transcription factor, was then co-transformed with various combinations of pGBKT7 and pACT2 constructs. An indication for interaction between fusion proteins was measured by β-galactosidase activity in extracts from the various transformants.
CKT2 was found to strongly interact with CK2α′ with a β-galactosidase activity of 5.8 ± 0.3 (Table 1). By contrast, CKT2 minimally interacted with the CK2α subunit, with β-galactosidase activity that is nearly 6-fold less than seen with the CKT2–CK2α′ interaction. CKT2 essentially did not bind to CK2β (Table 1). Since the yeast two-hybrid system showed that 13 yeast colonies contained CK2β, it suggested that CKT2 is a true target protein of CK2α′ since CK2α′ interacts with CK2β. This is also supported by the observation that clones of CKT2 and CK2β appeared simultaneously after co- transformation in S. cerevisiae strain AH109 (data not shown).
Table 1.
CK2α′ interacts with CKT2 in yeast two-hybrid assay
| Construct | Growth in plates |
β-Gal activity | Filter color | |
|---|---|---|---|---|
| Trp-/Leu- | Trp-/Leu-/His-/Aden- | |||
| BD-CK2α′ | + | + | 5.8 ± 0.3 | Blue |
| BD-CK2α | + | + | 1.0 ± 0.3 | Light blue |
| BD-CK2β | + | – | ≤0.05 | White |
β-galactosidase activities are expressed as the mean of five experiments ± SD. *P < 0.005 compared to BD-CK2α′.
To investigate which region of CKT2 binds to CK2α′, we constructed the full length, N-terminus and C-terminus portions of cDNA as binding domains. A co-transformation was performed with CK2α′ as the active domain in S. cerevisiae strain AH109 for β-galactosidase filter and liquid assay. The β-galactosidase activity was >2-fold greater with the C-terminus region (BD-CKT2 138–276) than the N-terminus region (BD-CKT2 1–137), and modestly higher than the full-length BD-CKT2 (Table 2). These data indicate that CK2α′ preferentially interacts with the C-terminus region of CKT2 but that there was modest binding to the N-terminus as well.
Table 2.
CKT2 interacts with CK2α′ in yeast two-hybrid assay
| Construct | Growth in plates |
β-Gal activity | Filter color | |
|---|---|---|---|---|
| Trp-/Leu- | Trp-/Leu-/His-/Aden- | |||
| BD-CKT2 | + | + | 5.2 ± 0.3 | Blue |
| BD-CKT2 138-276 | + | + | 6.5 ± 0.3 | Blue |
| BD-CKT2 1-137 | + | + | 2.8 ± 0.3 | Blue |
β-galactosidase activities are expressed as the mean of five experiments ± SD.
CKT2 binds to CK2α′ but not to CK2α in vitro
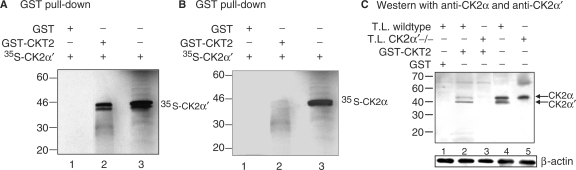
In a second approach to determine if CK2α′ interacts directly with CKT2, a GST pull-down assay was performed. Two methods were used. In the first method, CK2α′ or CK2α were translated under the T7 promoter and labeled with 35S-methionine. Labeled 35S-CK2α′ or 35S-CK2α were then incubated with GST-CKT2 fusion protein and glutathione-sepharose beads. After washing the beads three times, GST was cleaved from the glutathione-sepharose beads. The supernatants were separated by SDS–PAGE and subjected to autoradiography. As shown in Figure 6A (lane 1), GST protein alone did not bind to 35S-labeled CK2α′. However, after incubating GST-CKT2 with 35S-CK2α′ and electrophoresis, autoradiography revealed two protein bands which corresponded to CK2α′ (Figure 6A, lane 2). The presence of two isoforms of CK2α′ is likely related to the presence of two methionine start codons during in vitro translation. The electrophoretic location of CK2α′ was confirmed by electrophoresing only 35S-CK2α′ (Figure 6A, lane 3). As shown in Figure 6B (lane 1), GST protein alone did not bind to 35S-labeled CK2α. In contrast to CK2α′ binding to CKT2, no bands were detected after incubating the GST-CKT2 fusion protein with 35S-CK2α (Figure 6B, lane 2). Figure 6B, lane 3 contains only the labeled 35S-CK2α. These data confirmed that CKT2 can bind to CK2α′ but not to CK2α.
Figure 6.
In vitro binding between GST-CKT2 protein and CK2α′. (A) Autoradiograph of SDS-PAGE following incubation of GST or GST-CKT2 immobilized on glutathione-sepharose beads with in vitro translated 35S-CK2α′; Lane 1, GST protein alone with 35S-CK2α′; Lane 2, GST-CKT2 fusion protein with 35S-CK2α′; Lane 3, only 35S-CK2α′. Standard protein molecular weight markers (kDa) are on the left. (B) Autoradiograph of SDS–PAGE following incubation of GST or GST-CKT2 immobilized on glutathione-agarose beads with in vitro translated 35S-CK2α; Lane 1, GST protein alone with 35S-CK2α; Lane 2, GST-CKT2 fusion protein with 35S-CK2α; Lane 3, only 35S-CK2α. Standard protein molecular weight markers are on the left. (C) Western blot with anti-CK2α and anti-CK2α′ antibodies. GST protein was incubated with testicular lysates (T.L.) from wild-type mice (lane 1). GST-CKT2 fusion protein was incubated with T.L. from wild-type mice (lane 2) or CK2α′ −/− mice (lane 3). Following incubation, the samples were immunoprecipitated with glutathione-agarose beads followed by western blot using combined anti-CK2α and anti-CK2α′ antibodies. Lanes 4 and 5 are T.L. from wild-type and CK2α′ −/− mice, respectively, also immunoblotted with both anti-CK2α and anti-CK2α′. Data shown are representative of three independent experiments.
In a second method to examine the binding of CKT2 to CK2α′ and/or CK2α subunits, 10 µg of GST or GST-CKT2 fusion protein were incubated with 50 µg of testicular lysates from either wildtype or CK2α′−/− mice (23), precipitated with glutathione-sepharose beads, and analyzed by western blot with combined anti-CK2α and anti-CK2α′ antibodies. As shown in Figure 6C, a western blot of whole testicular lysates confirmed the presence of both CK2α and CK2α′ in the wildtype mice (lane 4) and only CK2α in the CK2α′−/− mice (lane 5). As also shown in Figure 6C (lane 1), GST protein alone did not bind CK2α or CK2α′ when GST was incubated with testicular lysates from the wild-type mice. Despite the fact that the relative band intensity for CK2α was greater than for CK2α′ in the testicular lysates from wild-type mice (lane 4), the band intensity for CK2α was weaker in the GST-CKT2 pulldown assay following incubation with testicular lysates from the wild-type mice. This suggests that the binding of CKT2 to CK2α is significantly weaker than to CK2α′. Importantly, no bands were observed when testicular lysates from CK2α′−/− mice were incubated with GST-CKT2 (lane 3). These results confirm the findings of the yeast two-hybrid and the biosynthetic GST-pull-down assays that CKT2 binds to CK2α′. In addition, it shows that CKT2 can also interact with CK2α but only in the presence of CK2α′.
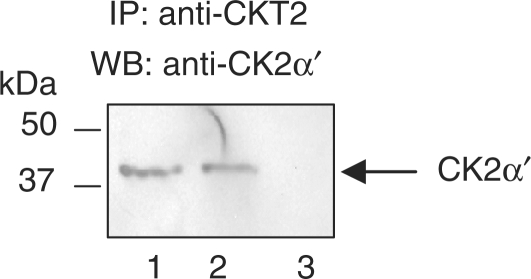
CKT2 binds to CK2α′ endogenously
We previously showed that the CK2α′ subunit of CK2 binds to recombinant CKT2. Using a third approach to confirm that CK2α′ binds to CKT2 endogenously, we determined whether CK2α′ co-immunoprecipitated with CKT2 in testicular lysates. Mice testicular lysates were incubated with anti-CKT2 antibody as described in Materials and methods section. As shown in Figure 7, immunoblot of testicular lysates with anti-CK2α′ antibody revealed a distinct band with a MW of ∼38 kDa (lane 1), consistent with CK2α′. Lysates immunoprecipitated with anti-CKT2 antibody revealed that CK2α′ co-immunoprecipitated with CKT2 (lane 2) whereas CK2α′ was not detected in lysates immunoprecipitated with a nonimmune IgG (lane 3).
Figure 7.
CK2α′ binds to CKT2 endogenously. Testicular lysates (T.L.) were immunoprecipitated with an anti-CKT2 antibody or a nonimmune antibody, followed by separation of the proteins by SDS–PAGE. The proteins were transferred onto nitrocellulose membranes and immunoblotted with anti-CK2α′ antibody. Lane 1, T.L.; lane 2, immunoprecipitation of T.L. with anti-CKT2 antibody; lane 3, immunoprecipitation with nonimmune IgG.
CK2 phosphorylates CKT2 in vitro and in vivo
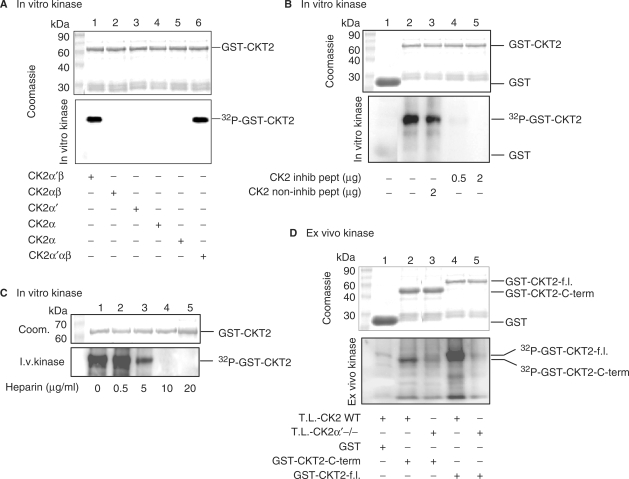
Based on the consensus sequence (S/T)XX(D/E) that is phosphorylated by CK2, CKT2 contains six potential CK2-mediated phosphorylation sites at positions 54SSTD, 68SLPE 85SKSE, 111SESD, 219SWLE and 227SSSD (Figure 1). Hence, we further substantiated the functional significance of CKT2-CK2 interaction by examining the phosphorylation of CKT2 by CK2 using three different approaches. First, to determine which subunit(s) of CK2 could phosphorylate CKT2, we incubated purified recombinant CKT2 with recombinant CK2α′β, CK2αβ, CK2α′, CK2α, CK2β or CK2α′αβ in an in vitro phosphorylation assay. As can be seen in Figure 8A (upper panel), a Coomassie stain showed equal loading of GST-CKT2 fusion protein. Incubation of CKT2 with CK2α′αβ served as a positive control and showed robust phosphorylation of CKT2 (Figure 8A, lower panel, lane 6). As also shown, neither CK2αβ, CK2α′, CK2α nor CK2β alone were capable of phosphorylating CKT2. However, CKT2-GST was phosphorylated by recombinant CK2α′β (lane 1), indicating that the regulatory subunit CK2β was required for CK2α′ kinase activity.
Figure 8.
CK2 phosphorylates CKT2 in vitro. (A) The ability of different subunit combinations of CK2 to phosphorylate CKT2 was examined in in vitro kinase assays in which CKT2 was incubated with recombinant CK2α′β, CK2αβ, CK2α′, CK2α, CK2β or CK2α′αβ in the presence of 5 μCi [γ-32P]-ATP. Aliquots were blotted to a membrane, and the amount of 32P incorporated into CKT2 was quantified with a PhosphoImager with the greatest phosphorylation band set at 100% (arbitrary units). A Coomassie stain of GST-CKT2 recombinant protein is shown in the top panel, the autoradiography in the bottom panel. Quantitation of CKT2 phosphorylation: Lane 1 (100%), Lane 2 (2%), Lane 3 (3%), Lane 4 (3%), Lane 5 (4%), Lane 6 (93%). (B) Phosphorylation of CKT2 by CK2 in the presence of a inhibitory peptide of CK2 or a noninhibitory peptide. GST (lane 1) or GST-full length CKT2 fusion protein (lanes 2–5) were incubated with recombinant CK2 (30 ng) in the presence of 5 μCi [γ-32P]-ATP as indicated. Some samples containing GST-full length CKT2 fusion protein and recombinant CK2 were also incubated in the presence of a noninhibitory peptide of CK2 (RETSALLRYFQTKFQNEKS) that does not contain a phosphorylation site (lane 3) or a competitive inhibitory peptide of CK2 (ETERRREEETEEE or DSDRRRDDDSDDD) that contains a phosphorylation site (lanes 4 and 5). The samples were subjected to SDS–PAGE and revealed by Coomasie (top panel) or autoradiography (bottom panel). Standard protein molecular weight markers are on the left. Quantitation of CKT2 phosphorylation: Lane 1 (5%), Lane 2 (100%), Lane 3 (88%), Lane 4 (15%), Lane 5 (5%). (C) Heparin inhibited CKT2 phosphorylation by CK2. Heparin at concentrations of 0.5–20 µg/ml was added to the in vitro phosphorylation assay between GST-CKT2 and recombinant CK2α′β. Quantitation of CKT2 phosphorylation: Lane 1 (100%), Lane 2 (100%), Lane 3 (25%), Lane 4 (12%), Lane 5 (7%). (D) CKT2 phosphorylation by testicular lysates (T.L.) of wild-type or CK2α′−/− mice. GST was incubated with T.L. from wild-type mice and subjected to ex vivo phosphorylation in the presence of [γ-32P]-ATP (lane 1). GST-C-terminus CKT2 (lanes 2 and 3) or GST-full length CKT2 (lanes 4 and 5) fusion proteins were subjected to ex vivo phosphorylation by T.L. from either wild-type (lanes 2 and 4) or CK2α′ −/− (lanes 3 and 5) mice. The samples were subjected to SDS–PAGE and revealed by Coomassie (top panel) or autoradiography (bottom panel). Quantitation of C-terminus-CKT2 phosphorylation: Lane 2 (100%), Lane 3 (22%). Quantitation of full length-CKT2 phosphorylation: Lane 4 (100%), Lane 5 (10%). Data shown are representative of three independent experiments.
Second, in vitro kinase assays were also performed with recombinant CKT2 and CK2 kinase in the presence of a competitive inhibitory peptide of CK2 that contains a phosphorylation site for CK2. A noninhibitory peptide without a CK2 phosphorylation site served as a control. A Coomassie stain showed equal loading of GST-CKT2 among experimental conditions (Figure 8B, upper panel). Whereas the control peptide did not significantly inhibited such phosphorylation (Figure 8B, bottom panel, lane 3), the CK2 inhibitory peptide strongly inhibited CK2 phosphorylation of CKT2 (Figure 8B, bottom panel, lanes 4 and 5). To confirm these results, in vitro phosphorylation of CKT2 with recombinant CK2 was performed in the presence of heparin, a polyanion known to inhibit CK2 activity (27). Shown in Figure 8C (top panel) is a Coomassie stain showing equal loading of GST-CKT2 fusion protein. Heparin inhibited CK2 phosphorylation of GST-CKT2 in a dose-dependent fashion, with ∼90% inhibition with 5 µg/ml of heparin and total inhibition with 10 µg/ml of heparin (Figure 8C, bottom panel).
Third, since the activity of CK2 is high in testes, we examined whether GST-CKT2 full length or GST-CKT2-C-terminus could be phosphorylated by testicular lysates from wildtype and CK2α′−/− mice in an ex vivo approach (21). Thus, mouse testicular lysates were used as a kinase source for the phosphorylation reactions. As shown in Figure 8D, GST protein alone was not phosphorylated by testicular lysates from the wild-type mice. As also demonstrated, both GST-CKT2 C-terminus and GST-CKT2 full length were robustly phosphorylated by testicular lysates from wild-type mice (lanes 2 and 4, respectively). These results indicate that CKT2 is phosphorylated by native cellular CK2 and that CK2α′ is the essential subunit for CK2 phosphorylation activity. Although there was minimal phosphorylation of the substrates by the testicular lystates from the CK2α′−/− mice (lanes 3 and 5, respectively), it suggests that kinases other than CK2α is responsible since the in vitro data with recombinant proteins showed that CKT2 was not phosphorylated by either CK2α or CK2αβ (Figure 8A).
To determine whether endogenous CKT2 can be phosphorylated by CK2, CKT2 was co-immunoprecipitated with CK2α′ from testicular lysates using anti-CK2α′ antibody and protein A-sepharose beads. After washing the beads, an in vitro CK2 kinase assay was performed by adding to the immunoprecipitate mixture [γ-32P]-ATP and CK2 casein (see Materials and Methods section). As shown in Figure 9A, immunoprecipitated CKT2 was phosphorylated by CK2 (lane 1). In contrast, testicular lysates incubated with nonimmune IgG showed no phosphorylation by CK2 (lane 2).
Figure 9.
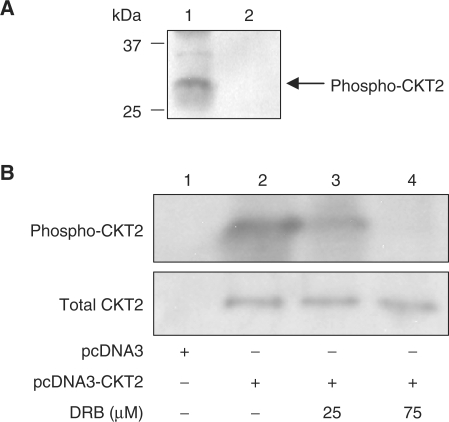
Endogeous CKT2 is phosporylated by CK2 in vitro and in vivo. (A) CKT2 was co-immunoprecipitated with CK2α′ from testicular lysates with anti-CK2α′ antibody (Lane 1). As a control, testicular lysates was also incubated with non-immune IgG (Lane 2), followed by an in vitro kinase assay using [γ-32P]-ATP and CK2. Data shown are representative of three independent experiments. (B) CKT2 is phosphorylated by endogenous CK2 in vivo. HEK293 cells were transfected with an empty vector or the CKT2 plasmid (pcDNA3-CKT2). After transfection and 24 hrs of culture, the cells were incubated with 1 mCi/mL [32P]-orthophosphate with or without a CK2 inhibitor DRB (5,6-dichloro-1-β-d-ribofuranosyl-benzimidazole) for 6 h. Following cell lysis and centrifugation, the supernatant was immunoprecipitated with anti-CKT2 antibody. The immunoprecipitate was separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and subjected to autoradiography. The membrane was then immunoblotted with anti-CKT2 antibody to determine equal loading of protein. Data shown are representative of three independent experiments.
To determine whether CKT2 can be phosphorylated endogenously by CK2 in vivo, we metabolically labeled CKT2-transfected HEK293 cells with [32P]-orthophosphate, followed by immunoprecipitation with anti-CKT2 antibody. In an analogous fashion, Li and co-workers (28) previously showed that HEK293 cells contain CK2 and have used this cell line to show that CK2 phosphorylates the transfected ARC protein in vivo. The immunoprecipitate was separated by SDS–PAGE and subjected to autoradiography. As shown in Figure 9B, HEK293 cells transfected with the empty vector did not reveal a phosphorylated band consistent in size with CKT2. However, in cells transfected with the plasmid that encodes for CKT2, phosphorylated CKT2 was detected in the immunoprecipitate. In the presence of a CK2 inhibitor (DRB), there was significant decrease in the cellular phosphorylation of CKT2. Immunoblotting of the same nitrocellulose membrane with anti-CKT2 revealed equal amounts of total CKT2. We conclude from these studies that CKT2 can be phosphorylated by CK2 both in an in vitro kinase assay as well as endogenously as detected by metabolic labeling.
DISCUSSION
CK2 is an intracellular signalling molecule known to phosphorylate many substrates (6). Indeed, it has been proposed that the number of proteins phosphorylated by CK2 may be much more numerous than those identified to date (6) and that CK2 alone may be responsible for the generation of a relatively large proportion (10–20%) of the eukaryotic phosphoproteome (10).
We identified a novel gene CKT2 whose product CKT2 is a target for CK2. RT-PCR and ISH studies showed that expression of CKT2 is testis-specific. Furthermore, immunohistochemistry and immunocytochemistry revealed that CKT2 is localized to the nuclei of male germ cells. Although CKT2 does not appear to have a nuclear localization signal (NLS) peptide, this sequence is not known to be required for proteins <45 kDa because they are able to passively diffuse through nuclear pore complexes (29).
Survey of the CKT2 sequence showed that it contains a coiled-coil domain and six putative CK2 phosphorylation sites, one of which is located in the coiled-coil domain. Phosphorylation of a coiled-coil assembly may serve to regulate the conformation of the parent protein (30). CKT2 was found to be phosporylated by recombinant purified CK2. GST pull-down and yeast two-hybrid assays showed that CKT2 binds to the CK2α′ subunit. Furthermore, in testicular lysates immunoprecipitated for CKT2, CK2α′ was co-immunoprecipitated, indicating that CK2α′ binds to CKT2 endogenously. In addition, we showed that endogenous CK2α′ immunoprecipitated from testicular lysates can phosphorylate CKT2. We also found that recombinant CKT2 is phosphorylated by CK2α′ but that the regulatory subunit CK2β was required for the kinase activity of CK2α′. As shown in Figure 1A, there are six amino acid residues on CKT2 that are potential sites of phosphorylation by CK2. Although phosphorylation of one or more of these sites is likely necessary for full CKT2 function, future studies involving site-directed mutagenesis of each or more of these residues would be required to determine the consequences of their phosphorylation in regulating spermatid chromatin condensation. Furthermore, it is interesting to speculate that the serine-rich domain from amino acid positions 164–182 of CKT2 may be phosphorylated by an unknown kinase, creating an acidic region that may render CKT2 to be more susceptible to phosphorylation by CK2 (Figure 1A).
A study of the spatio-temporal dynamics of CK2 in living cells has revealed that both CK2α and CK2α′ are located mostly in nuclei (31). Immuno-electron microscopy revealed that CKT2 is present in spermatid nuclei undergoing chromatin condensation, consistent with previous findings in yeasts that CK2-induced gene expression are those that encode chromatin remodeling proteins (32).
CK2α′ is highly expressed in testis (33) and appears to have an important role in survival and nuclear integrity of male germ cells (23,24). Genetic disruption of CK2α′ results in extensive nuclear alterations and apoptosis of differentiating male germ cells. Such morphologic changes in male germ cell differentiation include large nuclear envelope protusions and chromatin degeneration (24). Our observation that CKT2 is highly expressed at a specific step of spermatid chromatin condensation suggests that CKT2 and CK2α′ are involved in chromatin regulation during its remodelling. In spermatids undergoing condensation, somatic histones are replaced by lysine-rich transition proteins and, in turn, transition proteins are replaced by relatively arginine- and cysteine-rich protamines (34,35), processes that indicate complex protein–DNA interactions (36).
The TSPY testis-specific protein has already been found to play a role in the development of testicular cancer and prostate cancer. Interestingly, TSPY is known to be involved in chromatin remodelling and to be dependent on CK2 phosphorylation for its entrance into nuclei (37). The unique testicular phenotype of CK2α′−/− mice raises the question of whether CK2α′ could contribute to the pathogenesis of male germ cell tumors. Current evidence indicate that elevated expressions of CK2α′ are found in a number of metastatic tumors (38). Nuclear localization of CK2α is associated with a poorer prognosis in human prostate cancer (14). The anti-apoptotic function of CK2 may contribute to its ability to participate in transformation and tumorigenesis. CK2 activates histone deacetylase in hypoxia-associated tumors; in addition, upon hypoxic treatment, both CK2α and CK2α′ shuttle into the nucleus where histone deacetylases are localized (39). Administration of a peptide that targets the acidic phosphorylation domain for CK2 substrates has an anti-tumor effect in the mouse (17). Furthermore, strategies to inhibit CK2 have been ongoing in preclinical trials (16). Although CKT2 shares some homology with the oncogene product c-Myc (Supplementary Figure 1), the relatively sparse expression of CKT2 in spermatogonia would argue against its role in the nuclear alterations seen in the spermatogonia of CK2α′−/− mice and in testicular cancers.
In summary, this study identified CKT2 as the first known target for CK2α′ during spermatogenesis. CKT2 expression was found to parallel chromatin remodelling, suggesting important nuclear functions of CK2α′ and CKT2 in germ cells. CK2α′−/− mice and future development of a CKT2−/− mice will be important models to extend these studies. Future work needs to also address whether CKT2 is a CK2α′ target in premeiotic male germ cells and various germ cell tumors. Such elucidation would be invaluable to the emerging work of generating male germ cells from embryonic stem cells (40), given the known similarities between the primordial germ cells and embryonic stem cells (41,42).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
McLaughlin Research Institute for Biomedical Sciences.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Drs P.X. Xu, W.M. Zheng and P.Z. Mao for their expert technical assistance.
REFERENCES
- 1.Dobrowolska G, Lozeman FJ, Li D, Krebs EG. CK2, a protein kinase of the next millennium. Mol. Cell Biochem. 1999;191:3–12. [PubMed] [Google Scholar]
- 2.Hilgard P, Huang T, Wolkoff AW, Stockert RJ. Translated Alu sequence determines nuclear localization of a novel catalytic subunit of casein kinase 2. Am. J. Physiol. Cell Physiol. 2002;283:C472–C483. doi: 10.1152/ajpcell.00070.2002. [DOI] [PubMed] [Google Scholar]
- 3.Ackermann K, Neidhart T, Gerber J, Waxmann A, Pyerin W. The catalytic subunit alpha′ gene of human protein kinase CK2 (CSNK2A2): genomic organization, promoter identification and determination of Ets1 as a key regulator. Mol. Cell Biochem. 2005;274:91–101. doi: 10.1007/s11010-005-3076-2. [DOI] [PubMed] [Google Scholar]
- 4.Canton DA, Litchfield DW. The shape of things to come: an emerging role for protein kinase CK2 in the regulation of cell morphology and the cytoskeleton. Cell Signal. 2006;18:267–275. doi: 10.1016/j.cellsig.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Krebs EG, Eisenman RN, Kuenzel EA, Litchfield DW, Lozeman FJ, Luscher B, Sommercorn J. Casein kinase II as a potentially important enzyme concerned with signal transduction. Cold Spring Harb. Symp. Quant. Biol. 1988;53 (Pt 1):77–84. doi: 10.1101/sqb.1988.053.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 7.Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I. CK2 as a positive regulator of Wnt signalling and tumourigenesis. Mol. Cell Biochem. 2005;274:63–67. doi: 10.1007/s11010-005-3078-0. [DOI] [PubMed] [Google Scholar]
- 8.Hermosilla GH, Tapia JC, Allende JE. Minimal CK2 activity required for yeast growth. Mol. Cell Biochem. 2005;274:39–46. doi: 10.1007/s11010-005-3112-2. [DOI] [PubMed] [Google Scholar]
- 9.Allende JE, Allende CC. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 10.Pagano MA, Cesaro L, Meggio F, Pinna LA. Protein kinase CK2: a newcomer in the ‘druggable kinome’. Biochem. Soc. Trans. 2006;34:1303–1306. doi: 10.1042/BST0341303. [DOI] [PubMed] [Google Scholar]
- 11.Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol. Histopathol. 2001;16:573–582. doi: 10.14670/HH-16.573. [DOI] [PubMed] [Google Scholar]
- 12.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62:6770–6778. [PubMed] [Google Scholar]
- 13.Wang G, Unger G, Ahmad KA, Slaton JW, Ahmed K. Downregulation of CK2 induces apoptosis in cancer cells—a potential approach to cancer therapy. Mol. Cell Biochem. 2005;274:77–84. doi: 10.1007/s11010-005-3077-1. [DOI] [PubMed] [Google Scholar]
- 14.Laramas M, Pasquier D, Filhol O, Ringeisen F, Descotes JL, Cochet C. Nuclear localization of protein kinase CK2 catalytic subunit (CK2alpha) is associated with poor prognostic factors in human prostate cancer. Eur. J. Cancer. 2007;43:928–934. doi: 10.1016/j.ejca.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Vilk G, Saulnier RB, St Pierre R, Litchfield DW. Inducible expression of protein kinase CK2 in mammalian cells. Evidence for functional specialization of CK2 isoforms. J. Biol. Chem. 1999;274:14406–14414. doi: 10.1074/jbc.274.20.14406. [DOI] [PubMed] [Google Scholar]
- 16.Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim. Biophys. Acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Perera Y, Farina HG, Hernandez I, Mendoza O, Serrano JM, Reyes O, Gomez DE, Gomez RE, Acevedo BE, Alonso DF, et al. Systemic administration of a peptide that impairs the protein kinase (CK2) phosphorylation reduces solid tumor growth in mice. Int. J. Cancer. 2008;122:57–62. doi: 10.1002/ijc.23013. [DOI] [PubMed] [Google Scholar]
- 18.Olsen BB, Boldyreff B, Niefind K, Issinger OG. Purification and characterization of the CK2alpha′-based holoenzyme, an isozyme of CK2alpha: a comparative analysis. Protein Expr. Purif. 2006;47:651–661. doi: 10.1016/j.pep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Allende CC, Allende JE. Promiscuous subunit interactions: a possible mechanism for the regulation of protein kinase CK2. J. Cell Biochem. 1998;(Suppl 30–31):129–136. Review. [PubMed] [Google Scholar]
- 20.Lou DY, Dominguez I, Toselli P, Landesman-Bollag E, O’Brien C, Seldin DC. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol. Cell Biol. 2008;28:131–139. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerra B, Siemer S, Boldyreff B, Issinger OG. Protein kinase CK2: evidence for a protein kinase CK2beta subunit fraction, devoid of the catalytic CK2alpha subunit, in mouse brain and testicles. FEBS Lett. 1999;462:353–357. doi: 10.1016/s0014-5793(99)01553-7. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Landesman-Bollag E, Channavajhala PL, Seldin DC. Murine protein kinase CK2: gene and oncogene. Mol. Cell Biochem. 1999;191:65–74. [PubMed] [Google Scholar]
- 23.Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha′ catalytic subunit. Nat. Genet. 1999;23:118–121. doi: 10.1038/12729. [DOI] [PubMed] [Google Scholar]
- 24.Escalier D, Silvius D, Xu X. Spermatogenesis of mice lacking CK2alpha′: Failure of germ cell survival and characteristic modifications of the spermatid nucleus. Mol. Reprod. Dev. 2003;66:190–201. doi: 10.1002/mrd.10346. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsh EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd. New York: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.Bai X, Chan ED, Xu X. The protein of a new gene, Tctex4, interacts with protein kinase CK2beta subunit and is highly expressed in mouse testis. Biochem. Biophys. Res. Commun. 2003;307:86–91. doi: 10.1016/s0006-291x(03)01118-5. [DOI] [PubMed] [Google Scholar]
- 27.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl KA. Current Protocols in Molecular Biology. Boston: Wiley-Interscience; 1990. [Google Scholar]
- 28.Li P-F, Li J, Muller EC, Otto A, Dietz R, von Harsdorf R. Phosphorylation by protein kinase CK2: A signaling switch for the caspase-inhibiting protein ARC. Mol. Cell. 2002;10:247–258. doi: 10.1016/s1097-2765(02)00600-7. [DOI] [PubMed] [Google Scholar]
- 29.Jans DA, Hubner S. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol. Rev. 1996;76:651–685. doi: 10.1152/physrev.1996.76.3.651. [DOI] [PubMed] [Google Scholar]
- 30.Burkhard P, Stetefeld J, Strelkov SV. Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 2001;11:82–88. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- 31.Filhol O, Nueda A, Martel V, Gerber-Scokaert D, Benitez MJ, Souchier C, Saoudi Y, Cochet C. Live-cell fluorescence imaging reveals the dynamics of protein kinase CK2 individual subunits. Mol. Cell Biol. 2003;23:975–987. doi: 10.1128/MCB.23.3.975-987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barz T, Ackermann K, Dubois G, Eils R, Pyerin W. Genome-wide expression screens indicate a global role for protein kinase CK2 in chromatin remodeling. J. Cell Sci. 2003;116:1563–1577. doi: 10.1242/jcs.00352. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Rich ESJ, Seldin DC. Murine Protein Kinase CK2a’: cDNA and genomic cloning and chromosomal mapping. Genomics. 1998;48:79–86. doi: 10.1006/geno.1997.5154. [DOI] [PubMed] [Google Scholar]
- 34.Oko RJ, Jando V, Wagner CL, Kistler WS, Hermo LS. Chromatin reorganization in rat spermatids during the disappearance of testis-specific histone, H1t, and the appearance of transition proteins TP1 and TP2. Biol. Reprod. 1996;54:1141–1157. doi: 10.1095/biolreprod54.5.1141. [DOI] [PubMed] [Google Scholar]
- 35.Zambrowicz BP, Harendza CJ, Zimmermann JW, Brinster RL, Palmiter RD. Analysis of the mouse protamine 1 promoter in transgenic mice. Proc. Natl Acad. Sci. USA. 1993;90:5071–5075. doi: 10.1073/pnas.90.11.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshioka H, Geyer CB, Hornecker JL, Patel KT, McCarrey JR. In vivo analysis of developmentally and evolutionarily dynamic protein-DNA interactions regulating transcription of the Pgk2 gene during mammalian spermatogenesis. Mol. Cell Biol. 2007;27:7871–7885. doi: 10.1128/MCB.00990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krick R, Aschrafi A, Hasgun D, Arnemann J. CK2-dependent C-terminal phosphorylation at T300 directs the nuclear transport of TSPY protein. Biochem. Biophys. Res. Comm. 2006;341:343–350. doi: 10.1016/j.bbrc.2005.12.190. [DOI] [PubMed] [Google Scholar]
- 38.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pluemsampant S, Safronova OS, Nakahama K, Morita I. Protein kinase CK2 is a key activator of histone deacetylase in hypoxia-associated tumors. Int. J. Cancer. 2008;122:333–341. doi: 10.1002/ijc.23094. [DOI] [PubMed] [Google Scholar]
- 40.West JA, Park IH, Daley GQ, Geijsen N. In vitro generation of germ cells from murine embryonic stem cells. Nat. Protoc. 2006;1:2026–2036. doi: 10.1038/nprot.2006.303. [DOI] [PubMed] [Google Scholar]
- 41.Aflatoonian B, Moore H. Germ cells from mouse and human embryonic stem cells. Reproduction. 2006;132:699–707. doi: 10.1530/REP-06-0022. [DOI] [PubMed] [Google Scholar]
- 42.Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev. Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.