Figure 4.
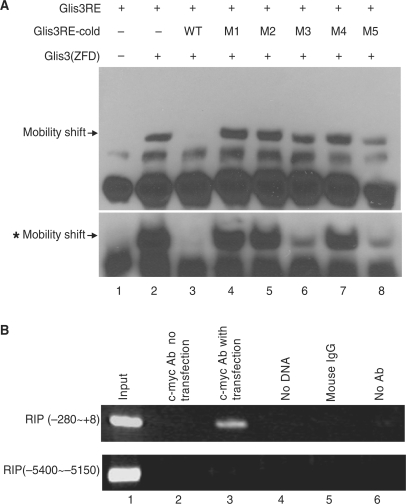
Glis3 binds to the RIP Glis3 response element (Glis3RE). (A) Synthetic biotinylated RIP sequence from −265 to −230, containing Glis3RE, was used as a probe in EMSA with an in vitro translated Glis3 ZFdomain [Glis3 (ZFD)] peptide. The specificity of the protein–DNA complex (lane 2) was confirmed by competition with a 2-fold molar excess of unlabeled wild-type competitor (lanes 3) but not a 2-fold molar excess of the mutants M1 to M5 (lanes 4–8). The mobility shift bands with asterisk showed the results of similar experiment using 20-fold molar excess wild-type or five mutant competitors. The specific bands are indicated by arrows. (B) The c-myc-Glis3–DNA complex was immunoprecipitated by c-myc antibody from 832/13 cells which had been transfected with c-myc-Glis3. Immunoprecipitated DNA was purified and analyzed by PCR using primers specifically spanning the Glis3RE region (RIP −280 to +8) or region remote from Glis3RE (−5400 to −5150). Lane 1, total input DNA (1:100 dilution); lane 2, DNA immunoprecipitated with c-myc Ab without c-myc-Glis3 transfection; lane 3, DNA immunoprecipitated with c-myc Ab with c-myc-Glis3 transfection; lane 4, no DNA template; lane 5, immunoprecipitation with mouse IgG; lane 6, DNA precipitated in the absence of antibody.