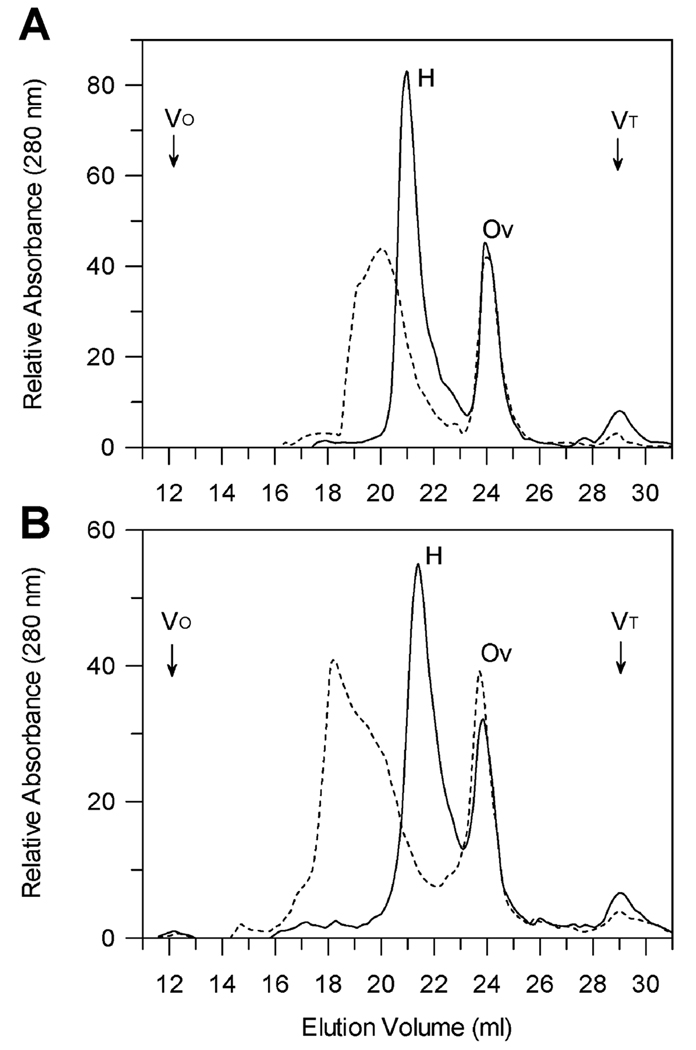
FIGURE 1.
Gel filtration chromatography of purified human factor H in the presence of dextran sulfate. Factor H was chromatographed in normal ionic strength PBS (Panel A) in the presence (dashed line) of 1 mg/ml dextran sulfate or in the absence of dextran sulfate (solid line) using ovalbumin as an internal standard. Samples of 0.3 ml containing 960 µg factor H and 730 µg ovalbumin with or without 1 mg/ml dextran sulfate (average m.w. approx. 5,000 providing an approximately 10-fold molar excess of dextran sulfate over factor H) were preincubated for 15 min at 21°C and loaded onto a 0.75 × 60 cm TosoHaas G4000SW column with a guard column equilibrated with PBS (10 mM sodium phosphate, 140 mM NaCl, 0.02% sodium azide, pH 7.4) (solid line) or PBS + 1 mg/ml dextran sulfate (dashed line). Chromatography was performed at 21°C at a flow rate of 0.5 ml/min. Panel B shows the results of similar chromatography using half ionic strength buffer (5 mM sodium phosphate, 70 mM NaCl, 0.01% sodium azide, pH 7.4). The positions of the column void volume (VO) and salt peak (VT) are indicated.